Abstract
Introduction: 9p24 contains genes that are critical for immune evasion and propagating cell division. The loci for PD-L1, PD-L2, JAK2, and the histone demethylases KDM4C/JMJD2C are linked on 9p24 (Van Roosbroeck et al. Genes, Chromosomes and Cancer 2016). Amplification or rearrangements of this region have been described in the pathogenesis of classical Hodgkin lymphoma (cHL) and primary mediastinal large B-cell lymphoma (Ansell et al. New England Journal of Medicine 2015). Additionally, JAK2 amplification up-regulates PD-L1 and L2, which leads to increased T-cell inactivation and suggests synergy between these drug targets. The recent success of PD-1 blockade in numerous malignancies has led to the development and approval of PD-1 inhibitors in cHL as well as other cancers. Targeted therapies are approved for JAK2 inhibition, such as ruxolitinib, and are in development for histone demethylases, which illustrates the utility of identifying the 9p24 amplicon in hematologic malignancies (HM) (Van Roosbroeck et al. Genes, Chromsomes and Cancer 2016). The goal of this analysis is to better understand the distribution of 9p24 abnormalities across a broader range of leukemias and lymphomas in order to facilitate future studies of targeted therapy.
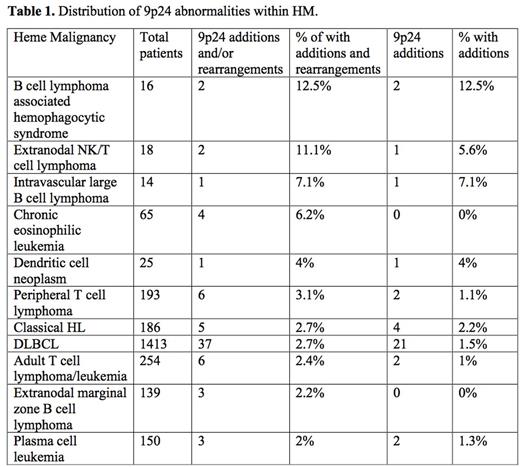
Methods: The National Cancer Institute's Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer was queried for 9p24 breakpoint abnormalities within HM. Incidence of additions and rearrangements in chromosome 9p24 for all subtypes of HM listed in the Mitelman Database were calculated. Individual references were manually reviewed and pathologic data was extracted as available from the primary sources. Diffuse large B-cell lymphoma (DLBCL) cases were further assessed for co-incident rearrangement of MYC (8q24), BCL2 (18q21) and BCL6 (3q27) with 9p24. All subtypes with greater than 2% incidence of additions and/or rearrangements in chromosome 9p24 were reported.
Results: 48,761 patients (pts) with HM across 74 lymphoid and myeloid subtypes were identified. 361 (<1%) pts had additions or rearrangements involving 9p24 across 53 subtypes of HM. 21 subtypes of HM had no 9p24 abnormalities. PTCL NOS had the highest incidence of aberrations (3.1% (6/193), 1.1% with additions and 2% with rearrangements) followed by cHL (2.7% (5/186), all additions) and DLBCL (2.7% (37/1413), 1.5% additions and 1.2% rearrangements) in malignancies with over 100 cases. Among DLBCL cases 9p24 was co-amplified/rearranged with MYC in 22% (8 cases), BCL6 27% (10), BCL2 14% (5) and in 3% (1) double-hit lymphomas. Minimal data was available on cell of origin; therefore, further analysis was not performed. No cases of DLBCL with 9p24 aberrations were T-cell rich DLBCL's (0/37). Among the rarer lymphomas, extranodal NK/T cell lymphoma (11%, 2/18) and intravascular B cell lymphoma (7%, 1/14) had higher incidences of 9p24 additions or rearrangements (Table 1).
Conclusion: Amplifications and rearrangement of 9p24 are rare in HM within the Mitelman database. Our study suggests that patients with DLBCL, adult T-cell leukemia/lymphoma, extranodal NK/T cell lymphoma, intravascular B cell lymphoma, peripheral T-cell lymphoma, and extranodal marginal zone B-cell may be considered for further studies with FISH and aCGH to further define the incidence of 9p24 alterations and potentially for targeted clinical trials. 9p24 abnormalities did not correlate with previously described phenotypic subtypes of DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal