Abstract
Background & aims
Tyrosine kinase inhibitors (TKI) still in a majority of cases do not cure chronic phase CML (CP-CML) and leave patients in a minimal residual disease state, requiring the indefinite continuation of TKI. However, in some patients, these agents are able to induce prolonged disease undetectability leading to TKI-cessation strategies and successful treatment-free remissions (TFR) in some. Stable (≥2 years) undetectability can be observed after Imatinib (IM), Nilotinib (NIL) and Dasatinib (DAS) and TKI-cessation strategies have been developed in these 3 settings. In most studies, the overall molecular relapse rate [defined as a loss of MMR, >0.1% BCR-ABL/ABLIS] is ~30-50% requiring the re-initiation of a TKI. The aim of this study is to characterize and compare the molecular relapse profiles in 3 cohorts of patients undergoing IM, NIL or DAS-cessation.
Methods
This is a bicentric observational retrospective analysis on a selected cohort of CP-CML patients fulfilling the following criteria: CP-CML since diagnosis; ≥2 years stable undetectable BCR-ABL (with ≥32,000 ABL copies/sample) evaluated on 4-6 consecutive follow-up samples, on IM, NIL, or DAS; first cessation whatever the reason for TKI prescription was; molecular relapse after TKI cessation defined as MMR loss on 1 occasion. Molecular analyses were performed in 2 reference laboratories belonging to the EUTOS/ELN quality control system. Relevant clinical data were extracted from the 2 databases of these centres, following the rules of our country regulations.
Results
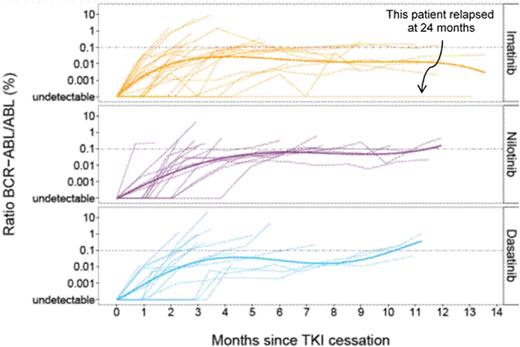
We identified 29 patients relapsing after IM, 15 after NIL and 14 after DAS cessation with a median age at TKI cessation of 57, 63, and 62.5 respectively (p=0.80). Interestingly, the male/female ratios were 1.41, 0.87 and 0.55 (p=0.38). Sokal score were respectively (L/I/H/NA): 10/12/6/2 for IM, 3/6/6/0 for NIL, 5/4/4/1 for DAS groups (overall p=0.78). Pre-TKI IFN-awas administered in 6 patients in the IM, 4 in the NIL and 1 in the DAS groups (p=0.42). ACA were present at diagnosis in 2, 1 and 1 patients respectively. Two patients had NIL and 7 DAS, in first-line. MR4.5 duration was 37 months for IM, 24.5 months for NIL and 33 months for DAS. Overall TKI duration until TKI cessation were 86 months for IM, 85 months for NIL and 70 months for DAS (p=0.28). The median interval between MR4.5 and TKI cessation was shorter for NIL (25 Months) than for DAS (33 months, p=0.041). The kinetics of relapse are shown in Figure 1 where the evolution of the BCR-ABL ratio by TKI has been smoothed with a polynomial regression.
Relapses occurred after a median of 3.94 (0.89-7.2) for IM, 5 (0.69-7.6) for NIL and 4 (2.87-7.52) months for DAS (p=0.86). Kaplan-Meier analysis of the molecular disease-free survival (DFS) according to TKI and since TKI cessation is surprisingly not different (log-rank test p=0.95) between the 3 TKI, suggesting that TKI2 do not slow down the kinetics of relapse. When looking at the KM estimates of molecular DFS since stable MR4.5, it seems longer with DAS than with NIL (long-rank test p=0.018), despite the fact that the duration of MR4.5 before cessation was significantly longer with NIL than with DAS. This might suggest a differential effect of these 2 compounds on the BCR-ABL+ stem cell compartment. TKI were re-initiated after a median of 0.9, 0.8 and 0.75 months after relapse for IM, NIL and DAS. After relapse 6 patients switched from IM to NIL, 1 from NIL to Peg-IFN-a, 4 from NIL to DAS, whereas all DAS restarted the same TKI. MR4.5 was regained after 6.5, 3.9 and 7.41 months in the IM, NIL and DAS groups with a median follow-up of 43, 23.75 and 25 months after relapse respectively. Two patients died, 1 in the IM group from terminal cardiac insufficiency and 1 in the DAS group from metastatic secondary tumor. At last follow-up, patients regained ≥ MMR in all groups except 1 (0.16% at 3 months DAS rechallenge).
Conclusion
Despite its obvious limitations (relatively low number of patients, retrospective non randomized study) this study characterizes the kinetics of molecular relapse after TKI cessation for TFR and might point out differences suggesting that in these patients the BCR-ABL+ cells responsible for relapse might be different, or that the differential kinase inhibition profile by each TKI induces a different behaviour at relapse. However, these data need to be confirmed ideally in a prospective setting.
Nicolini:Ariad, BMS: Consultancy, Speakers Bureau; Novartis: Speakers Bureau. Dulucq:Novartis: Speakers Bureau. Mahon:Pfizer: Honoraria; Ariad: Honoraria; Novartis: Honoraria, Research Funding; BMS: Honoraria. Etienne:BMS: Speakers Bureau; Pfizer: Speakers Bureau; ARIAD: Speakers Bureau; novartis: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal