Abstract
BACKGROUND: BCR/ABL1 transcripts ≥ 10% after 3 months or 6 months was an effective prognosic indicator for long-term outcomes (Hanfstein B,et al. Leukemia,2012). The incidence of disease progression was significantly higher in patients who failed to achieve Early molecular response (EMR) rates-BCR/ABL1 transcripts ≥ 10% by 3 months or 6months (Hughes T,et al.Blood,2003).During 2009-2016,there have 429 patients (Pts) with standard dose Imatinib 400mg QD for first line in our centre,but 29.3% (102 Pts) BCR/ABL1 transcripts ≥ 10% after 3 months and 31.3% (77 Pts) BCR/ABL1 transcripts ≥ 1% after 6 months.
OBJECTIVES: Here, 3 years datas from our centre of NIL 400mg BID (n=43) as secondline vs. high-dose IM 600mg QD (n=71) for standard-dose IM 400mg QD in newly dignosed CML-CP who failed to EMR were analysed to evaluate the molecular response rate and safety.
METHODS: Inclusion Criteria:Patients of age ≥ 18,newly dignosed CML-CP (within 6 months of dignosis).BCR/ABL1 transcripts ≥ 10% at 3 or 6 months with standard dose IM 400mg QD. ECOG performance status ≥ 2. And normal hepatic,renal and cardiac function. Study Design: Datas of NIL 400mg BID (n=43) as secondline vs. high-dose IM 600mg QD (n=71) for standard-dose IM 400mg QD (n=114) in newly dignosed CML-CP who failed to EMR were analysed to evaluate by Sokal risk. Primary endpoints are cumulative incidence rates of MMR and rate of patients achieved MMR by Sokal risk. Secondary endpoints are OS and PFS by Sokal risk and safety.
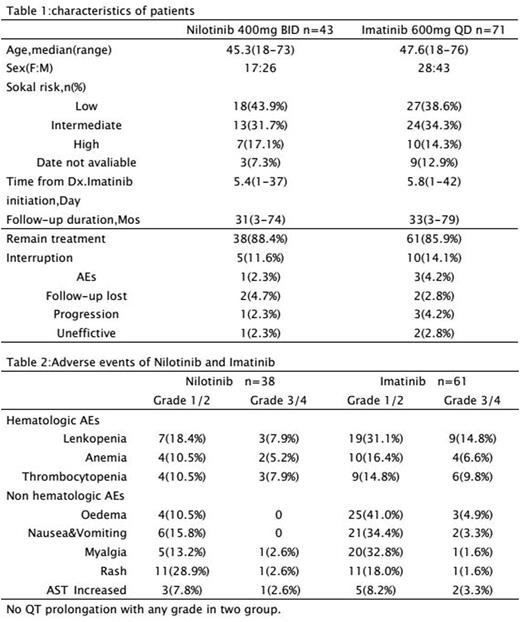
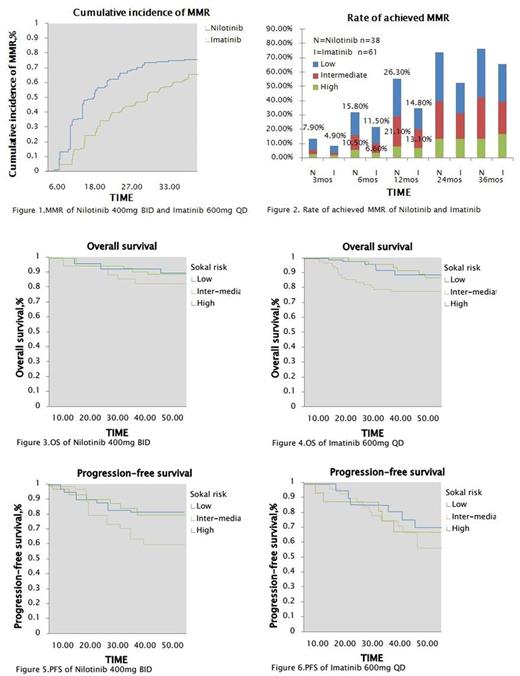
RESULT: Two study groups were balanced with age, gender and Sokal risk score (Table 1). Rates of cumulative incidence of MMR in two groups were 13.2% in NIL vs. 3.3% in IM respectively (P=.0010) at 3 months,34.3% vs. 14.8% respectively (P=.0042) at 6 months and 55.3% vs.34.4% respectively (P=.0143) at 12 months but there were not significantly different between two groups at 24 and 36 months (Figure 1). Rate of patient achieved MMR in study were 7.9% (n=3) in NIL vs. 4.9% (n=3) in IM respectively (P=.0087) at 3 months,15.8% (n=6) vs. 11.5% (n=7) respectively (P=.0076) at 6 months and 26.3% (n=10) vs. 14.8% (n=9) respectively (P=.0084) at 12 months in low risk group by Sokal risk score; 10.5% (n=4) vs. 6.6% (n=4) respectively (P=.0009) at 6 months and 21.2% (n=8) vs. 13.1% (n=8) respectively (P=.0041) at 12 months in intermediate risk group (Figure 2). PFS of two groups were 80.3% vs. 70.2% respectively in low risk (P=.0013) and 78.6% vs. 66.8% respectively in intermediate risk (P=.0051) at 60 months but there were not significantly different in high risk (Figure 5,6). The OS of two groups were not significantly different in all Sokal risk at 12,36 and 60 months also (Figure 3,4). The most common (rate of AEs ≥ 10%) hematologic AEs were grade 1/2 in two groups but AEs rate of NIL was less than IM.Nonhematologic AEs also focused on grade 1/2, included rash (28.9%,n=11) and myalgia (13.2%,n=5) in NIL; oedema (41.0%,n=25) and nausea & vomiting (34.4%,n=21) in IM commonly, but there were not significantly different between two groups. Grade 3/4 AEs were uncommon in hematology & nonhematology (Table 2).
CONCLUSION: NIL resulted more rapidly to achieve MMR compared with high dose IM who failed to EMR with standard dose IM. Low ang Intermediate groups of Sokal risk achieved significantly higher MMR rate at 6 and 12 months and higher PFS at 60 months in patients receiveing NIL compared with IM but OS similarly in all groups. The safety profiles of NIL and IM were different and the hematologic AEs of NIL was less than IM. The result of this study support that NIL can achieve molecular response rapidly for BCR/ABL1 transcripts ≥ 10% with Imatinib (IM) 3 or 6 months in CML-CP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal