Abstract
Introduction: The MPN are clonal hematopoietic stem cell (HSC) disorders characterized by an overproduction of blood cells and an increased risk of transformation to an aggressive phase with myelofibrosis (MF) and/or acute myeloid leukemia (AML). Polycythemia vera (PV) is the most common clinical subtype, and while PV starts as an indolent process, nearly 25% of patients will progress to MF and/or AML. PV is caused by acquired mutations of JAK2, yet JAK2 mutations alone do not account for MF or AML transformation. Mutations in genes encoding epigenetic regulators are associated with MPN transformation, but the mechanism of action is not understood. HMGA1/2 chromatin binding proteins are potent oncogenes that drive tumor progression by activating oncogenic and stem cell transcriptional networks. Both HMGA1/2 are overexpressed in acute leukemia and have been shown to be drivers of clonal expansion in myeloid disease in humans and in murine myeloproliferative disease models. We hypothesized that HMGA proteins could be critical drivers of transformation in PV and therefore tested the association of HMGA1/2 expression to transformation in human and murine PV.
Methods: We examined the HSC genomic context and clonal evolution in 49 JAK2V617F-positive PV patients using standard and SNP-array karyotyping and a targeted resequencing panel of 163 genes associated with myeloid cancers. We examined HSC clonal burden by examining JAK2V617F HSC genotypes on a single cell basis. We measured HMGA1 and HMGA2 expression in a JAK2V617F positive human cell line, in isolated CD34+ HSCs from PV patients during chronic and transformation phases, in JAK2V617F transgenic murine models of PV (tgJAK2V617F) and PV-AML (tgJAK2V617F/MPLSV; Blood 2015;126:484) using a real-time quantitative RT-PCR (qRT-PCR) assay.
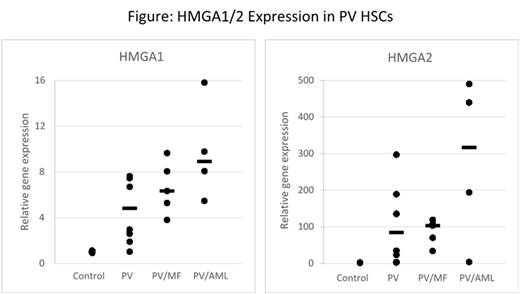
Results: Both HMGA1 and HMGA2 mRNA were up-regulated in all JAK2V617F-positive contexts. In primary human PV CD34+ HSCs, HMGA1 and HMGA2 were found to be increased by 7 and 100 fold, respectively, compared to controls. Moreover, there was a dramatic up-regulation in both HMGA1/2 in patients who transformed from PV to MF or AML compared to chronic phase PV, whether analyzed cross-sectionally (Figure) or prospectively in selected patients. In addition to disease phase, over-expression of HMGA1/2 correlated with clonal dominance of JAK2V617F-homozygous stem cells, and additional mutations of epigenetic regulators including EZH2 and SETBP1. Similarly, when assessed in unfractionated bone marrow or in tumor samples in the two transgenic mouse models for PV and PV-AML, Hmga1/2 were overexpressed compared to wild-type littermates, with highest levels in the PV-AML transgenic mouse model.
Conclusion:HMGA1 and HMGA2 are overexpressed in PV, and higher levels associate with disease progression to MF and AML, both in human PV and in transgenic murine models of PV. These data suggest HMGA proteins are critical drivers of PV transformation and that the mechanism of HMGA1/2 overexpression is a consequence of aberrant JAK/STAT signaling and epigenetic dysregulation. Our findings indicate that HMGA1/2 overexpression may function as a necessary molecular switch for PV leukemic transformation. Therefore, HMGA proteins and their transcriptional pathways offer novel therapeutic targets aimed at the prevention of PV progression to MF and AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal