Abstract
Successful engraftment of hematopoietic stem and progenitor cells (HSPCs) during bone marrow transplantation requires appropriate homing and retention of transplanted cells in the bone marrow (BM) and the activation of a proliferative program in response to signals from the hematopoietic microenvironment (HM). Molecular pathways regulating migration, homing and retention of HSPCs in the BM are integrated by RhoGTPasesincluding Rac and CDC42, however the complex cues that drive the proliferative response of these cells following transplantation are less clear.
We have previously described the hematopoietic phenotype of adult mice lacking Vav1, a multi-domain, hematopoietic-specific GEF for Rac and CDC42. Deletion of Vav1 does not affect steady state hematopoiesis in adult mice, but severely compromises the engraftment potential of HSPCs. In the absence of Vav1, signal transduction from SDF1α is impaired in HSPCs, leading to abnormal localization and reduced retention of these cells in the HM, a phenotype similar to deficiency of Rac (Sanchez-Aguilera et al. PNAS, 2011). Here, we define an unexpected role for Vav1 in mediating post-irradiation proliferative responses of HSPCs.
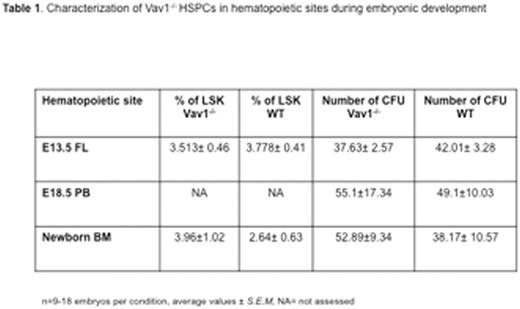
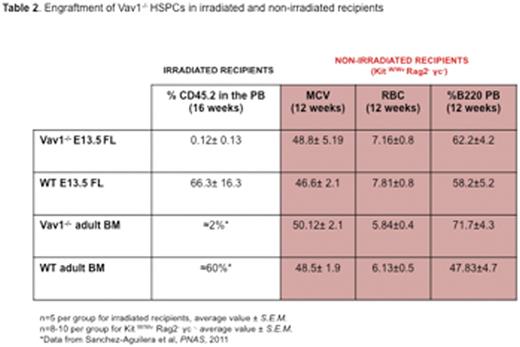
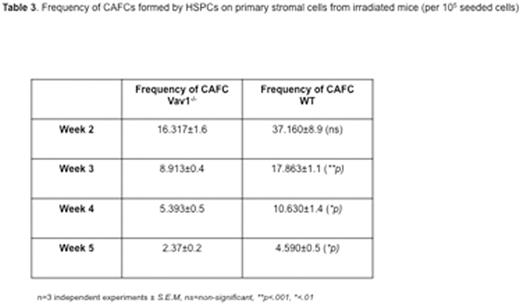
Surprisingly, we observed that deletion of Vav1 does not affect HSPC migration during ontogeny, a process largely mediated by SDF1α. The number of immunophenotypically and functionally defined HSPCs in Vav1-/- E13.5 fetal liver (FL) was comparable to WT (Table 1). Similarly, no difference was detected in HSPCs in the peripheral blood (PB) of E18.5 embryos or in the BM of newborn mice. However, similar to adult cells, Vav1-/- fetal HSCPs showed severely defective engraftment in lethally irradiated recipients (see Table 2). In marked contrast, both adult and fetal Vav1-/- HSCPs could engraft non-irradiated (Kit W/Wv Rag2- γc-) recipients, achieving successful correction of the macrocytic anemia and B cell leukopenia phenotype of recipient mice (Table 2, in red). Reduced proliferation of Vav1-/- HSPCs was also observed in vitro upon co-culture with primary irradiated stromal cells (Table 3). No differences among genotypes were detected when using non-irradiated stromal cells. These data suggest a distinct role for Vav1 in mediating responses of HSPCs to the HM after irradiation.
To further clarify this phenotype, we investigated the role of individual soluble factors on proliferative responses of Vav1 HSPCs. Given their known role in expansion and proliferation of hematopoietic progenitors we focused on gp130 cytokines. We found that both IL-6 and IL-11, prominent members of this cytokine family, were increased in the BM of irradiated WT recipients, compared to both non-irradiated WT recipients (3x and 2x increase) and Kit W/Wv Rag2- γc- (2x and 3x increase) age- and sex-matched animals. To validate the potential role of IL-6 and IL-11 in Vav1 function, we stimulated HSPCs with both cytokines and observed that they induced phosphorylation of Vav1 and activation of Rac, but not CDC42. Using CFU assays, liquid culture experiments and BrdU analysis we confirmed that deletion of Vav1 abolishes the proliferative responses elicited by IL-6 and IL-11 on HSPCs in vitro.
In summary, we show that Vav1 acts in HSPCs to mitigate responses to pro-inflammatory cytokines present in the HM during engraftment following irradiation. Manipulating the gp130-Vav-Rac axis in HSPCs could represent a strategy to enhance engraftment of normal cells in conditioned recipients.
Williams:bluebird bio: Research Funding; Novartis: Consultancy; Orchard Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal