Abstract
Introduction: Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in the Western world. In the US, there was an estimated 14,620 new cases of CLL and 4650 deaths due to CLL in 2015; the incidence is 4.5 per 100,000 based on 2008-2012 data. CLL is a disease of the elderly; the median age at diagnosis in the US is 71 years, whereas only 11% of patients are younger than 55 years. Median survival ranges from 2 to over 10 years depending on a patient's medical conditions and disease characteristics. Combination chemotherapy with fludarabine, cyclophosphamide, and rituximab (FCR) is the standard of care first-line therapy for CLL without comorbidities. Bendamustine plus rituximab (BR) is recommended in patients not eligible for FCR. The purpose of the current analysis is to examine differences in healthcare utilization between two cohorts of newly diagnosed CLL patients undergoing BR or FCR therapy. Moreover, we analyzed differences between the cohorts across age groups.
Methods: Newly diagnosed CLL patients between 1/1/2005-10/31/2015 treated with first-line BR or FCR were identified from the Truven Health MarketScan® Research Databases. Inclusion criteria were: age ≥18 years, continuous enrollment from 6 months prior to 1 month after index date (e.g., first prescription for BR/FCR), and no other CLL prescription prior to the index date. Healthcare utilization variables included number of outpatient visits, emergency room (ER) visits (yes/no), and hospitalizations (yes/no), and were calculated on a per-month basis from the index date through end of available data as well as every 6 months up to 218 months. Nonparametric tests, logistic regression, and general linear models were used to test for differences while controlling for baseline variables. Analysis examined main effects of cohort and age as well as the interaction between the two.
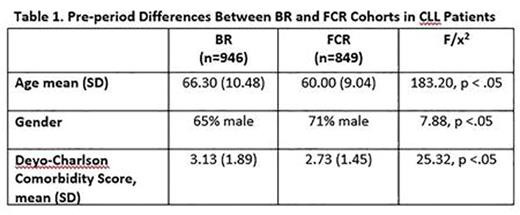
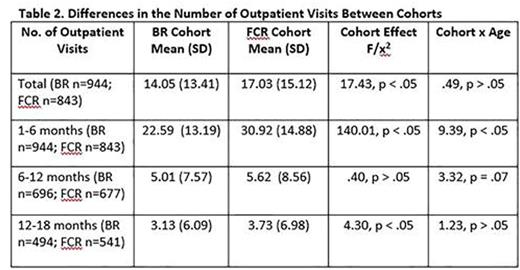
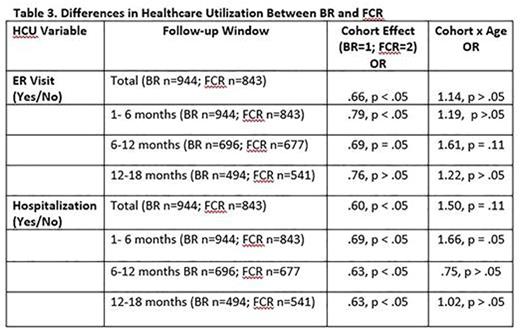
Results: Of 1795 CLL patients (male = 68%; mean age = 63.33 years, SD = 10.32) identified, 946 were in the BR cohort and 849 patients were in the FCR cohort. Baseline differences are presented in Table 1. The BR cohort was significantly older, comprised more females, and had more frequent comorbid conditions relative to FCR patients. As shown in Table 2, the BR cohort also experienced significantly fewer outpatient visits over the course of enrollment during the first 6 months of therapy and during months 12-18. The BR cohort was consistently less likely to experience an ER visit or hospitalization than the FCR cohort across all follow-up periods (Table 3). Differences between the cohorts were particularly salient for the FCR patients who were 70 years or older, who experienced, on average, more outpatient visits as well as had a greater likelihood of an ER visit or hospitalization stay.
Conclusion: The results of this analysis suggest that, in general, the healthcare utilization of CLL patients that remain on BR is significantly lower than patients who remain on FCR. Patients aged ≥70 receiving FCR experienced significantly more days of hospitalization, outpatient visits, and ER visits than patients of the same age treated with BR. These results support the emerging saliency of BR as an effective and safe treatment option for elderly CLL patients.
Gabriel:Teva Pharmaceuticals, Inc.: Employment. Szabo:Eli Lilly & Company; Zoetis: Equity Ownership; Patient Centered Outcomes Research (PCORI): Consultancy; Teva Pharmaceuticals, Inc.: Employment. Lo-Coco:Teva, Lundbeck: Honoraria, Speakers Bureau; Teva, Novartis, Baxalta, Pfizer: Consultancy. Tang:Teva Pharmaceuticals, Inc.: Employment. Pathak:Teva Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal