Abstract
Background: Congenital hemolytic anemias (CHAs) are a heterogeneous group of inherited RBC disorders including membrane and enzyme defects and dyserythropoietic anemias. Iron overload is a well recognized complication of hereditary hemoglobinopathies, both in transfusion-dependent and independent cases. However, little is known in congenital hemolytic anemias, with the exception of anecdotic reports in pyruvate kinase deficiency and dyserythropoietic anemias.
Aim: to describe the clinical and hematological features at diagnosis and enrolment, to investigate iron overload by hepatic and cardiac T2* MRI, and to study inflammatory/regulatory cytokine profiles (IL-6, TNF-alpha, IFN-gamma, IL-10, IL-17) and hepcidin levels in patients with CHAs. Confounding factors such as hemocromatosis genotyping, metabolic syndrome, and hepatic viral profile were also considered.
Methods: Between July 2015 and April 2016, 38 patients were enrolled (13 hereditary spherocytosis -HS, 3 hereditary stomatocytosis - HSt, 8 congenital dyserythropoietic anemia type II - CDAII, 13 pyruvate kinase deficiency - PKD, 1 glucose-phosphate isomerase deficiency). HS cases were enrolled on the basis of ferritin >300 ng/mL at diagnosis. Cytokine levels were detected in serum by ELISA. Comparisons were made by Students T test (continuous) and Fisher's exact test (categorical), and correlations by Pearson's linear coefficient.
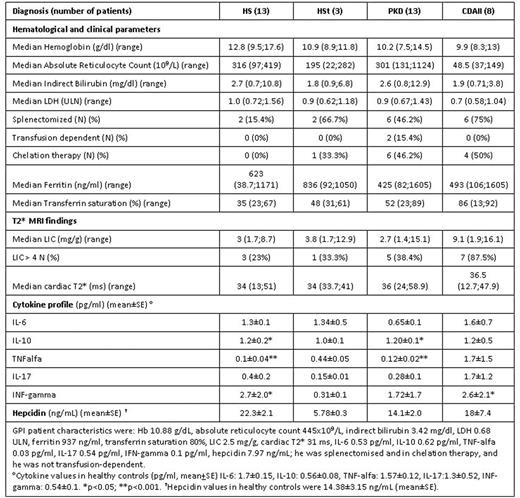
Results: The main clinical and hematological findings are shown in table. Median Hb values progressively decreased in the 4 groups considered, being close to normal in HS and moderately reduced in CDAII patients, whereas hemolytic parameters were comparable among groups. Consistently with clinical severity, ferritin values were particularly high in CDAII (together with transferrin saturation-TfS) and PKD patients, notwithstanding chelation in about half cases of both groups. Of note, only 2 PKD patients were transfusion-dependent, suggesting that other factors are involved in iron overload. Splenectomy had been performed in 17/38 (44.7%), mainly CDAII. Liver iron concentration (LIC) showed a great heterogeneity in all groups, with a trend towards higher values in CDAII; 16/36 (44%) patients had a LIC>4 mg/g DW (23%, 33%, 38% and 88% in HS, HSt, PKD and CDAII, respectively). Cardiac T2* value was normal in all subjects, with the exception of a HS and a CDAII case. Regarding possible cofactors, 12/16 displayed at least one of the following: 1 homozygous for HFE C282Y and 1 for H63D mutations, 3 HCV+, 4 BMI>25, 2 alcohol abuse, 3 heterozygous for HFE mutations. The following positive correlations were observed at enrolment: LIC and ferritin (r=0.68, p<0.05), LIC and TfS (r=0.34, p=0.05), and cardiac T2* and TfS (r=0.34, p<0.05). Moreover Hb values at diagnosis negatively correlated with LIC (r=0.37, p<0.05). Interestingly, among the 28 cases with ferritin <800 ng/mL, 10 (36%) displayed liver iron overload (LIC>4), of whom 5 with the above listed cofactors. As regards cytokine levels, IL-10 was significantly increased in HS, PKD and CDAII groups compared with normal cases; TNF-alpha was decreased in HS and PKD, and IFN-gamma increased in HS and CDAII. Ferritin values were positively correlated with IL-6 and IFN-gamma, and TfS negatively with IL-6 (r= -0.38, p<0.05). Hepcidin values were slightly increased in CHAs compared with normal controls, and correlated positively with ferritin (r=0.33; p<0.05), and negatively with TfS (r= -0.56; p<0.001). Finally, hepcidin levels were positively correlated with IL-6 (r=0.62; p<0.001), and negatively with IFN-gamma (r=0.3; p<0.05).
Conclusion: Iron overload is highly prevalent in CHAs, particularly in PKD and CDAII, is independent from transfusion support, and is also observed in cases with ferritin <800 ng/mL. T2* MRI is the gold standard approach to evaluate iron overload in CHAs (as in other congenital anemias) and its use is advisable, particularly in the presence of cofactors, for early chelation. Cytokine studies suggest the existence of a positive loop among ferritin, hepcidin, and inflammatory cytokines such as IL-6 and IFN-gamma, and of a negative loop among TfS, hepcidin, and the same inflammatory cytokines. These findings disclose important hints to understand the multiple biological mechanisms of iron overload, and support the rationale for new emerging therapies.
Barcellini:Agios: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal