Abstract
Introduction
Crohn's disease and Ataxia Telangiectasia have been managed by extended release of dexamethasone from autologous red blood cells (RBC) with encapsulated dexamethasone sodium phosphate DSP. The EryDexSystem (EDS) is an automated system that loads RBC ex vivo using hypotonic opening of RBC followed by hypertonic resealing of the RBC and washing to prepare the DSP-RBC for infusion. In vivo, DSP is dephosphorylated within the RBC to dexamethasone which passively diffuses into the plasma. The objective of this two-phase study was to elucidate pharmacokinetics (PK) and in vivo 24-hour recovery of RBCs as well as RBC survival (T50) properties of RBC encapsulated DSP.
Materials and Methods
The study was conduct in two separate Phases, A and B. Phase A, the 24-hour RBC recovery and T50 survival phase, was designed as a randomized, concurrently controlled, single-blind, single-center study to determine the in vivo kinetics of EDS-processed autologous RBC. Healthy volunteer consenting subjects were randomized to receive autologous RBCs prepared using EDS and loaded with either 15-20mg DSP (Group 1A) or sham hypotonic saline (Group 2A). EDS prepared RBC were radiolabeled with 51-Cr following standard methods, and the in vivo labeled RBC followed over 49 days post infusion.
The Phase B PK study was designed as an open-label, single-center Phase I study that evaluated two dose levels of DSP encapsulated in RBCs using the EDS. Healthy volunteer consenting subjects were randomized to receive autologous RBCs loaded with either 2.5-5 mg DSP (Group 1B) or 15-20 mg (Group 2B). Post-infusion plasma levels of dexamethasone were followed (over 42 days).
Both studies conformed to the Declaration of Helsinki.
Results
Phase A: Ten subjects (3male; 7 female) were randomized to Groups 1A or 2A. The mean 24-hour RBC recovery ± SD [95% CL] was 77.9 ± 3.3% [73.8-81.9%] and 72.7 ± 10.5% [57.8-85.7%] for Groups 1A and 2A, respectively. The mean ± SD RBC life span in Group 1A was 84.3 ± 8.3 days with a mean T50 of 42.1 ± 4.1 [95% CL: 37.0, 47.3] days, whereas these values were 88.9 ± 6.2 days and 44.4 ± 3.1 [95% CL: 40.6, 48.3] days, respectively, in Group 2A. Sixteen (16) treatment-emergent adverse events (TEAEs) were recorded in Group 1A and 23 in Group 2A. All TEAEs were judged to be unlikely related to the treatment.
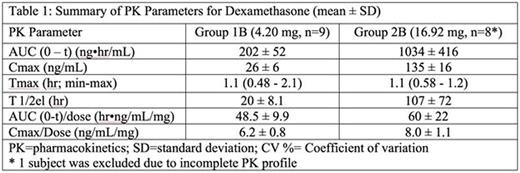
Phase B: Eighteen subjects (12 male; 6 female) were randomized to Groups 2A and 2B. The actual DSP loading doses (mean ± SEM) were 4.2±0.27 mg and 16.9±0.90 mg. Release of dexamethasone from RBCs in vivo peaked at 1 hour after the end of IV infusion independent of the dose. A detailed summary of the PK parameters for dexamethasone for each treatment group is shown in Table 1. A sustained release of dexamethasone could be detected until 14 and 35 days after the single IV infusion ofEryDex in Group 1B and 2B, respectively. Six (6) TEAEs were reported in each group and were judged to be unlikely related to the study drug or procedure.
Conclusion
The results for the mean RBC in vivo recovery for DSP-loaded EDS-processed cells meet the FDA criteria for 24-hour RBC recovery of ≥ 75%, without adverse impact on the survival of EDS-processed RBCs. Most of the dexamethasone was rapidly released from the RBCs in vivo with a maximum peak occurring 1 hour after the end of the intravenous infusion, independent of the dose administered, but sustained release of dexamethasone could be detected until 14 and 35 days post infusion for the low and high doses, respectively. DSP-loaded autologous RBCs prepared using the EDS delivered a sustained dose of dexamethasone in vivo. Additional efficacy studies in targeted patient populations are indicated.
Szczepiorkowski:EryDel S.P.A.: Research Funding. Ferrari:EryDel S.P.A.: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Benatti:EryDel S.P.A.: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Mambrini:EryDel S.P.A.: Employment, Equity Ownership. Hartman:EryDel S.P.A.: Consultancy. Dumont:EryDel S.P.A.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal