Abstract
Introduction: Transfusion related sepsis is a serious concern for both military and civilian trauma centers. The primary source of bacterial contamination associated with such events is room temperature (RT) stored platelets. Due to high risk of bacterial contamination, RT stored platelets may only be administered up to 5 days post-collection. Although approved by the FDA, cold stored platelets may only be administered 3 days post-collection under current regulations. In this study, we utilized the opportunistic pathogen Acinetobacterbaumannii - a reported cause of platelet contamination and important hospital acquired infection (HAI) - as a model organism to assess bacterial growth in platelets stored either at room temperature (22oC) or refrigerated (4oC), as well as determine contributors to bacterial growth.
Methods: Apheresis platelets in plasma (PLT) were obtained from healthy donors using the Terumo Trima Accel Automated Blood Collection System (Terumo BCT). Platelet poor plasma (PPP) was obtained from PLT aliquots centrifuged twice at 2,500 x g for 5 min. Other PLT aliquots were stimulated with Phorbol 12-myristate 13-acetate (PMA) to generate activated platelets (aPLT). Following activation, aPLTs were pelleted through centrifugation at 2,500 x g for 5 min and the releasate (REL) was transferred to a separate container. Pelleted aPLTs were washed 3 times in sterile PBS and suspended in PPP. In some experiments aliquots of PPP were supplemented with 40 mM lactic acid. Aliquots of PLT, PPP, aPLT, and REL were transferred to pH SAFE minibags (Blood Cell Storage, Inc) and inoculated (a.k.a., contaminated) with A. baumannii clinical isolate Ci79. Minibag aliquots stored at RT were agitated using an orbital shaker set to 60 rpm while refrigerated aliquots were stored under static conditions. Bacterial growth was monitored daily through dilution plating. In some experiments, lactate levels in PLT aliquots were assessed by iSTAT (Abbott) using CG4+ test cartridges.
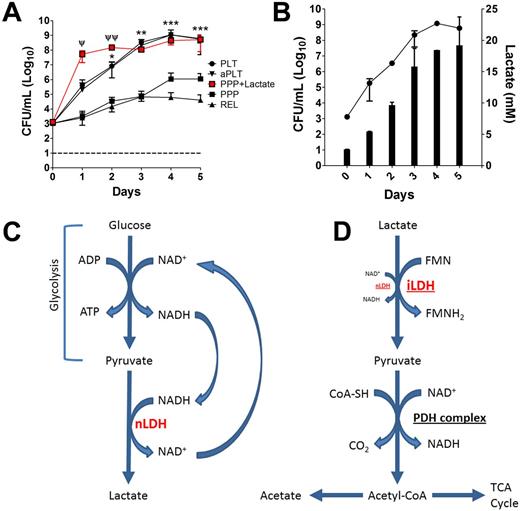
Results: Bacterial growth progressed exponentially over the first 3 days post-collection in PLT aliquots stored at RT. However, growth was significantly (p < 0.05) reduced in PPP units (Fig. 1A). Neither platelet activation status nor released factors appeared to have any effect as no significant differences were observed in bacterial growth between contaminated PLT and aPLT units, nor between contaminated PPP and REL units (Fig. 1A). Growth progressed at a much faster rate and to a greater magnitude in the presence of live platelets (PLT and aPLT), suggesting the contribution of platelet metabolism. Thus, lactate levels were assessed in PLT units and found to mirror bacterial growth (Fig. 1B). Furthermore, addition of lactic acid to RT stored PPP restored bacterial growth (Fig. 1A). Growth remained static throughout under all treatment conditions stored refrigerated.
Conclusions: Bacterial growth remained static over the 5 day post-collection observation period during cold storage. Additionally, bacterial growth at RT appeared to be related to increased production of lactate from pyruvate via NAD-dependent lactate dehydrogenase (nLDH) following glycolysis (Fig. 1C). To that end, many bacteria, including A. baumannii,as well as various Staphylococcus spp, and Streptococcus spp, possess genes encoding for NAD-independent lactate dehydrogenases (iLDH) which enable utilization of lactate as a carbon source (Fig. 1D). These data demonstrate that not only can bacterial growth be controlled through refrigeration, but RT stored platelets potentiate bacterial growth through their accelerated metabolism relative to cold storage.
Lactate Promotes Bacterial Growth at Room Temperature in Stored Platelets. Bacterial growth under various conditions (PLT ●, aPLT ▼, PPP + Lactate ■, PPP ■, or REL ▲) during RT storage (A). Lactate production mirrors bacterial growth over time (B). Simplified schematic detailing platelet lactate production (NAD-dependent lactate dehydrogenase = nLDH) (C). Utilization of lactate by bacteria as a carbon source (NAD-independent lactate dehydrogenase = iLDH; pyruvate dehydrogenase complex = PDH complex) (D). Error bars represent ± SD. Statistical differences determined by student's t-test. Statistical differences between PLT and PPP; * = p < 0.05, ** = p < 0.005, *** = p < 0.0005. Statistical differences between PLT and PPP + Lac; Ψ = p < 0.05, Ψ Ψ = p < 0.005.
Lactate Promotes Bacterial Growth at Room Temperature in Stored Platelets. Bacterial growth under various conditions (PLT ●, aPLT ▼, PPP + Lactate ■, PPP ■, or REL ▲) during RT storage (A). Lactate production mirrors bacterial growth over time (B). Simplified schematic detailing platelet lactate production (NAD-dependent lactate dehydrogenase = nLDH) (C). Utilization of lactate by bacteria as a carbon source (NAD-independent lactate dehydrogenase = iLDH; pyruvate dehydrogenase complex = PDH complex) (D). Error bars represent ± SD. Statistical differences determined by student's t-test. Statistical differences between PLT and PPP; * = p < 0.05, ** = p < 0.005, *** = p < 0.0005. Statistical differences between PLT and PPP + Lac; Ψ = p < 0.05, Ψ Ψ = p < 0.005.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal