Abstract
DBA is a rare bone marrow failure syndrome clinically defined by onset of hypoproliferative anemia in early childhood accompanied by a grossly normocellular bone marrow. With age, a significant fraction of patients progress to pancytopenia and a hypocellular bone marrow, with some increased risk of progression to leukemia. Autosomal dominant or sporadic heterozygous mutations in genes encoding for ribosomal subunit proteins have been etiologically linked to DBA. How loss of function mutations in ribosomal proteins results in selective loss of erythroid progenitors with variable severity is incompletely understood. Chronic transfusion therapy and corticosteroid treatment can alleviate anemia, but both cause severe long term toxicities. Hematopoietic stem cell transplantation, when available, is the only definitive treatment modality for the hematological manifestations, and the only known treatment for DBA patients once pancytopenia develops.
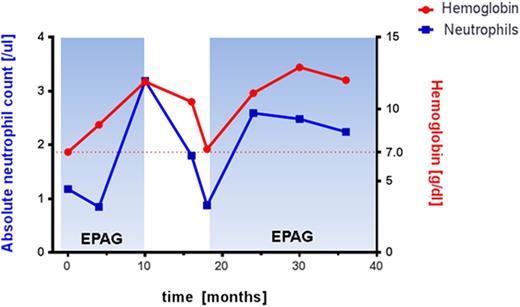
Here we report on a 28 year old female patient diagnosed with DBA at age of one month based on clinical criteria. Recent mutational analysis identified a novel mutation in intron 4(c.356+3>C) of the RPS19 gene affecting a known splice donor site that has previously reported in DBA patients. She did not respond to corticosteroids and was maintained on transfusions. She failed various experimental therapies including danazol, hematopoietic growth factors including IL-3 and GM-CSF, and anti-thymocyte globulin/cyclosporine without sustained clinical response. After treatment with daclizumab in 2006 her transfusion requirements decreased for about 4 years. In 2012 she presented with progressive pancytopenia and hypocellular bone marrow without overt dysplasia, and normal cytogenetic analysis. She required red cell transfusions. The patient was enrolled in a clinical research protocol investigating the efficiency and safety of the thrombopoietin receptor agonist eltrombopag (EPAG) in patients with moderate aplastic anemia or those with bone marrow failure with unilineage cytopenia (ClinicalTrials.gov: NCT01328587). The study is designed as a non-randomized, phase II, dose modification study with the primary endpoint hematological response at 16 weeks. EPAG was administered at 50mg daily and escalated every 2 weeks by 25 mg to the maximal dose of 300mg daily. At response assessment the patient's hemoglobin (Hgb) improved from 7.0g/dl to 8.9g/dl and platelets from 112K/ul to 171k/ul. She required no further transfusions, and was deemed a responder. EPAG was subsequently continued at the same dose level for an additional 6 months when the peripheral cells counts peaked at Hgb 11.9g/dl, platelets 251K/ul, ANC 3.19 K/ul, and EPAG was discontinued for protocol-defined robust response. After discontinuation of EPAG the patient's hemoglobin steadily declined over a period of 8 months. EPAG was reinitiated at 300mg per protocol. Peripheral blood cell counts rapidly improved and the EPAG dose was slowly tapered. The patient is currently on EPAG 50mg daily with normal blood cell counts. We have not observed any EPAG related toxicities in our patient, and repeated cytogenetic studies on bone marrow documented a normal karyotype.
To the best of our knowledge this is the first report documenting EPAG efficacy in a DBA patient. Trautman et al. (2012) previously reported a DBA patient that failed treatment with low dose EPAG after six months. Our observation warrants further systematic investigation of EPAG treatment for DBA patients within a clinical research study setting.
Winkler:GSK/Novartis: Research Funding. Townsley:Novartis: Research Funding. Desmond:Novartis: Honoraria. Dumitriu:GSK/Novartis: Research Funding. Grasmeder:GSK/Novartis: Research Funding. Young:GSK/Novartis: Research Funding. Dunbar:GSK/Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal