Abstract
Background: Successful immunotherapeutic approaches for acute myeloid leukemia (AML) have yet to be developed. We hypothesized that targeting both the innate and adaptive immune responses in leukemic hosts would elicit significant anti-tumor activity with lesser toxicities than chemotherapy. To test this, we evaluated the efficacy of immune checkpoint inhibition (murine anti-PD-1 antibody (ab)) alone and in combination with 5,6-dimethylxanthenone-4-acetic acid (DMXAA), an innate immune agonist and anti-vascular agent, in an immunocompetent model of murine AML.
Methods: Expression of PD-L1 was assessed by flow cytometry on the murine AML cell line, C1498, alone and following treatment with vehicle, DMXAA or interferon-gamma (positive control). A LEGEND MAX mouse ELISA kit was utilized to measure IL-6 and IFN-β. C57BL/6 mice were inoculated with stably transfected C1498 murine AML cells expressing luciferase and the fluorescent protein DSRed2. Once disease was established, animals were treated with vehicle, DMXAA (20 mg/kg every four days x 7 weeks), anti-murine PD-1 antibody (10 mg/kg every 3 days x 4 doses) or DMXAA + anti-PD-1 antibody (same doses). Animals underwent weekly clinical assessments, weights, and bioluminescent imaging for disease burden. Overall study endpoints were time to morbidity and differences in leukemia disease burden as compared with vehicle-treated controls. Mice were euthanized on day 15 after injection of C1498 cells (8 days following treatment) for collection of plasma, bone marrow, liver and spleen samples for tumor burden, activated T-cells.
Results: DMXAA doses (ranging from 1-100 μg/ml) inhibited C1498 in vitro cell growth at 48 hours (48h) in a dose dependent manner. Treatment of C1498 cells in culture with escalating doses of DMXAA (1-100μg/ml) or IFN-gamma (positive control) induced higher PD-L1 expression on these AML cells consistent with direct immunomodulatory effects. Furthermore, C1498 cells exposed to higher doses of DMXAA (10-100μg/ml) for 48h produced measurably higher levels of IL-6 and IFN-β expression in cell supernatants.
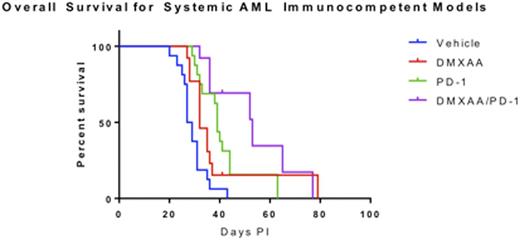
We then examined the effects of DMXAA, anti-PD-1 ab, or the combination of DMXAA + anti-PD-1 ab treatment in vivo in C57BL/6 mice systemically engrafted with C1498-luciferase AML cells. Treatment overall was well tolerated and resulted in significantly decreased disease burden as measured by total body bioluminescence vs. vehicle controls (p<0.05). Median time to morbidity was significantly decreased in all treatment arms as compared with controls: vehicle = 28 days, DMXAA = 32 days, anti-PD-1 ab = 39 days, and combination DMXAA + anti-PD-1 ab = 53 days (p<0.05). Combination therapy resulted in significantly longer overall survival than single agent therapy (DMXAA vs. DMXAA+anti-PD-1 ab, p=0.032; anti-PD1 ab vs. DMXAA+antii-PD-1 ab p=0.038)(n=total 13-16 mice per group) (representative data shown in Figure 1). Therapy with DMXAA alone and in combination with anti-PD-1 ab was associated with markedly higher PD-1, PD-L1, and PD-L2 expression levels in bone marrow cells harvested from leukemic mice 48h after treatment. Significantly higher numbers of activated T cells were also identified in the bone marrow and spleen of leukemic mice following two weeks of DMXAA therapy alone or in combination with anti-PD-1 ab. Additional in vivo measurements of systemic cytokine levels following therapy are underway.
Conclusions: Here we demonstrate that the combination of an innate immune agonist (DMXAA) with an immune checkpoint inhibitor (anti-PD-1 ab) improved anti-leukemic effects in a preclinical AML model. In vitro DMXAA therapy inhibited murine AML growth in a dose dependent manner, enhanced PD-L1 expression, and induced leukemic production of cytokines (IL-6, IFN-β). In vivo combination DMXAA and anti-PD-1 ab therapy in an immunocompetent murine AML model increased activated host T cell numbers and marrow PD-1/L1/L2 expression in conjunction with decreased tumor burden and prolonged overall survival. These studies may pave the way for future clinical trials evaluating this novel immunomodulatory strategy in AML patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal