Abstract
Background: Minimal residual disease (MRD) in B lymphoblastic leukemia (B-ALL) as measured by flow cytometry is well-established as an important prognostic factor; Its presence is used to adjust treatment in most therapeutic protocols in children , while the lack of a standardized assay has hampered the introduction of flow cytometric MRD in adult ALL trials. On the other hand, measuring MRD has become part of the standard of care even for patients not on clinical trials. Although flow cytometric analysis of MRD in B-ALL has been well standardized in clinical trials of the Children's Oncology Group (COG) in North America (Borowitz et al Blood 2015;126:964), there are no data on performance characteristics of this assay within routine clinical labs.
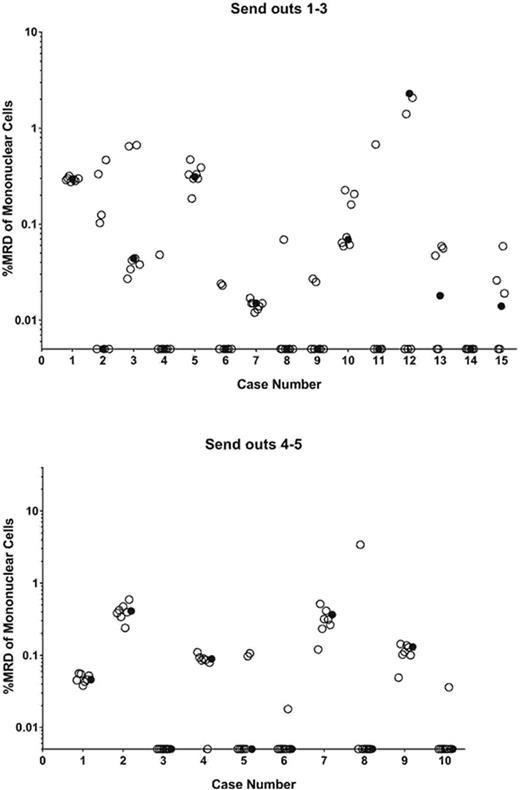
Methods: As part of an ongoing effort to standardize and decentralize ALL MRD measurement, list-mode data from post-induction marrows were distributed from one COG reference lab to 7 different clinical flow cytometry labs self-identified as having experience with ALL MRD. All labs were provided with the COG protocol used for MRD analysis along with a template illustrating recommended gating strategies, and formulas for calculating MRD burden. List-mode files of pre-treatment B-ALL samples analyzed with the standard COG B-ALL MRD antibody panel were distributed for comparison. In the first rounds, list-mode files from 15 samples were distributed to the 7 labs. Samples included those with and without MRD as assayed in the reference lab. Samples were selected to include normal B-cell precursors (hematogones) or MRD that had undergone phenotypic change with therapy. Some samples had artifacts that could potentially mimic small MRD populations. To improve performance, educational sessions were implemented, and 10 more list-mode file samples were distributed in a second round of challenges.
Results: There was considerable dispersion of MRD results among the 7 labs that analyzed the list-mode files (Fig 1A). Although high levels of MRD were uniformly recognized, several labs misclassified normal B-cell precursors and/or mischaracterized small artifacts as MRD. Moreover, among samples correctly identified as positive, quantitative differences in MRD levels from those reported by the reference lab were seen. Among 95 total challenges, the overall discordance rate was 24%. This included 11 false positives, 7 false negatives, and an additional 5 quantitatively discordant cases among positives (defined as outside +/- 0.5 log of the reference lab value). In the second round, positive and negative samples, as well as those with normal precursors were included, though these samples contained fewer artifacts than those of the first round. Performance improved considerably (Fig 1B); out of 70 challenges, there were 5 false positives and 1 false negative (8.6% discordance), and no cases were quantitatively discordant. Four of the 6 deviations occurred in a single lab. Three samples with hematogones were still misclassified as MRD.
Conclusions: Despite the provision of a standardized analysis protocol, even experienced laboratories have difficulty with B-ALL MRD analysis by flow cytometry. Some of these difficulties can be overcome with education, but even with education recognition of hematogones still remains a challenge for some labs. Extrapolating these results to other laboratories with less experience indicates the need for caution in migrating MRD testing from centralized reference laboratories, and suggests that implementation of MRD testing as part of routine clinical management of B-ALL patients in a manner similar to that of routine flow cytometric classification of leukemia may require additional training and resources.
Wood:Pfizer: Honoraria, Other: Laboratory Services Agreement; Juno: Other: Laboratory Services Agreement; Amgen: Honoraria, Other: Laboratory Services Agreement; Seattle Genetics: Honoraria, Other: Laboratory Services Agreement. Lozanski:Stemline Therapeutics Inc.: Research Funding; Beckman Coulter: Research Funding; Boehringer Ingelheim: Research Funding; Genentech: Research Funding. Mukundan:CCS Associates: Employment. Higley:CCS Associates: Employment. Sigman:CCS Associates: Equity Ownership. Borowitz:BD Biosciences: Research Funding; Medimmune: Research Funding; Bristol Myers Squibb: Research Funding; HTG Molecular: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal