Abstract
Introduction
Mantle cell lymphoma (MCL) which relapses or becomes refractory to frontline chemotherapy can be a clinical challenge. There have been several targeted agents approved in relapsed MCL including bortezomib, lenalidomide and ibrutinib (IBR) with the best single-agent responses seen with IBR. IBR is an oral, Bruton tyrosine kinase (BTK) inhibitor which has an overall response rate (ORR) of 67% with a median duration of response of 17.5 months in relapsed MCL (Wang, Blood 2015). While these responses are impressive in this population, only 1/3 of patients will have a complete response and only 1/3 of responding patients will have a 24 month PFS. Thus, improvements are needed.
Venetoclax (VEN) is an oral selective BCL2 inhibitor which is currently FDA approved in relapsed 17p-deleted chronic lymphocytic leukemia. We and others have shown synergistic cytotoxicity with VEN and IBR (Axelrod Leukemia 2014) which prompted us to explore the combination in a Phase I/Ib clinical trial (clinicaltrials.gov ID: NCT02419560). This study was supported by a grant from AbbVie Inc.
Methods
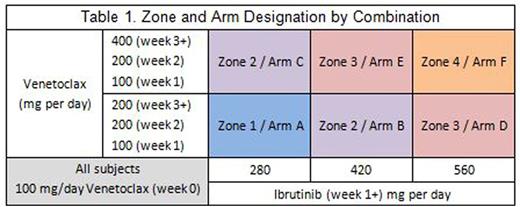
Given overlapping toxicities with VEN and IBR, namely neutropenia and GI toxicities, potential for drug-drug interactions given both are metabolized by CYP3A, and a wide range of therapeutic dosing for the two drugs, a dose finding study is appropriate. A continual-reassessment model was designed to test six dosing strategies (table 1). Subjects start treatment with single agent VEN at 100mg PO daily and increase to the allocated dose per table 1. After 1 week of VEN, subjects start the allocated dose of IBR. Subjects are monitored closely for tumor lysis syndrome (TLS) and hospitalized for TLS monitoring when starting IBR. Subjects are treated with the combination for 6 months and are encouraged to continue IBR after that time.
The study enrolls in 2 stages. In the first stage, subjects are enrolled one at a time to sequential arms. Up to 2 subjects are allowed on each arm in a zone before enrollment in the zone is paused. Subsequent zones are enrolled once at least one subject in every arm of the zone does not have a DLT during the DLT window. The second stage begins when a subject has a DLT or all arms have enrolled at least 1 subject. In the second stage, subsequent subjects are allocated to an arm based on DLT's and ORR at 2 months occurring in prior patients on the study, thus the study aims to find the optimal dosing combination of IBR and VEN for both toxicity and response. Enrollment will continue until 10 subjects are allocated to an arm or 28 total subjects are enrolled.
Eligible patients must have documented relapsed MCL after at least 1 line of chemotherapy. Subjects must not have bulky disease, no evidence of TLS, and must not have been previously treated with IBR.
Results
Enrollment began 10/2015 and at the time of submission, we have treated 8 subjects and have finished stage I of the study. Subjects were enrolled on arms A to E. Mean age is 63 years (range 49-81). 7 of the 8 subjects are male. 5 subjects were refractory to their prior treatment and 3 subjects have progressed after an autologous bone marrow transplant.
Seven of the 8 subjects are evaluable for adverse events. 5 subjects have completed the 6-week DLT window. There have been 15 related adverse events reported with 14 of these being grade 1 or 2. No TLS has been reported. One DLT at arm E was identified (grade 4 neutropenia) which prompted us to move to stage II of the study.
Three subjects (arm A, B, and C) are evaluable for response with all achieving at least a partial response. One subject on arm C, had a complete response at 4 months of the combination.
Conclusion:
Early results suggest tolerability for the combination of IBR and VEN in Relapsed MCL. There have been no signs of TLS, though subjects with high risk for TLS are excluded. One DLT (neutropenia) has been reported in Arm E and thus modeling will start to find the optimal combination using both toxicity and response. Responses have been seen across the various treatment arms. Continual re-assessment modeling is an adequate study design for combination studies with targeted agents to identify optimal dosing, accounting for both toxicity and response.
Portell:Infinity: Research Funding; Roche/Genentech: Research Funding; Acerta: Research Funding; AbbVie: Research Funding. Chen:Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Millenium: Consultancy, Research Funding, Speakers Bureau; Genentech: Consultancy, Speakers Bureau; Merck: Consultancy, Research Funding. Cohen:Millennium/Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kahl:Seattle Genetics: Consultancy; Celgene: Consultancy; Infinity: Consultancy; Gilead: Consultancy; Juno: Consultancy; Pharmacyclics: Consultancy. Williams:Jansen: Research Funding; Pharmacyclics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal