Abstract
Background CML is a clonal disorder characterized by the presence of the Philadelphia (Ph) chromosome which encodes for the bcr-abl tyrosine-kinase (TK). Target therapy with the TK inhibitors (TKIs)) has greatly improved its outcome.
Treatment with second generation TKIs - e.g. nilotinib (NIL) - results in deeper and faster responses and prevents disease progression. Sustained responses may enable TKI discontinuation. However, even in the event of qPCR negativity, a fraction of patients (pts) experience disease recurrence possibly due to persistence of quiescent leukemic stem cells (LSCs).
Degree and mechanisms of LSCs clearance during TKI treatment are not established yet and conflicting results are reported in the literature. Work from the group of Bocchia (Bocchia 2008; Defina 2012) showed reduction of LSCs during long term imatinib (IM) therapy; moreover, in CCyR pts residual LSCs are more rarely detected after NIL compared to IM therapy and, in a small fraction of pts this occurs after very short-term NIL therapy.
This data conflicts with in vitro evidence that NIL is not superior to IM in inducing growth suppression in CML LSCs (Konig, 2008).
To verify the in vivo activity and time-course of first-line NIL therapy on bone marrow (BM) Ph+ stem cells (CD34+/lin-) clearance, on behalf of the Rete Ematologica Lombarda (REL) the PhilosoPhi34 study (EudraCT: 2012-005062-34) was designed.
Primary efficacy endpoint was to measure the rate of BM residual CD34+/lin-Ph+ cells in CCyR pts at 6 months of treatment.
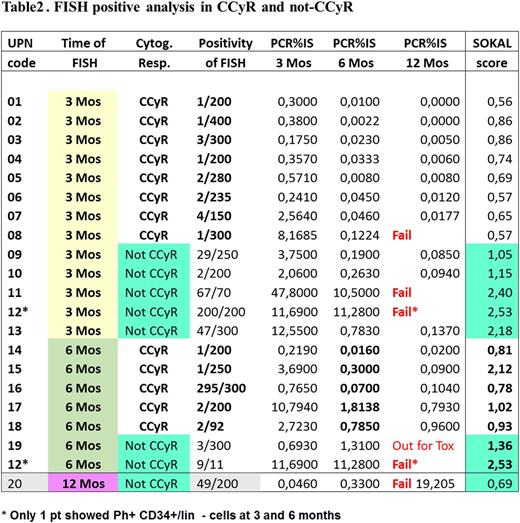
Methods BM cells were collected and stored at diagnosis and at 3,6 and 12 mos of treatment. CD34+/lin- cells were purified using a Diamond CD34 Isolation Kit Miltenyi (97% of purity). FISH analysis of selected unstimulated CD34+/lin- cells was performed according to standard procedures; considering the low sensitivity of the test, in order to define the test as negative at least 200 nuclei were examined.
The A'Hern single stage design was chosen for the present study; considering the CCyR results obtained in the ENESTnd study and the anticipated number of un-evaluable tests, a minimun of 87 pts were required.
Results Enrolment of the 87 pts was completed by June 2015. Table 1 summarises pts' characteristics and response to treatment. FISH results are as follows:
at 3 mos, 8/65 (12,3%; CI 95%: 2,3%-15,7%) evaluable FISH tested positive (10 negative tests not evaluable);
at 6 mos 5/71 (7%; CI 95% :2,3-15,7%) evaluable FISH tested positive (7 negative tests not evaluable);
at 12 mos, 0/68 (0%; CI95%:0,0-5,2%) evaluable FISH tested positive (9 negative tests not evaluable).
At any time point, Sokal score did not predict for FISH results. However, as outlined in Table 2, H-Sokal score pts are less prevalent among pts who achieve a CCyR, a requirement for FISH analysis.
Of the 4 pts who failed the treatments' objectives by 12 mos, 1 was in CCyR with detectable residual CD34+/lin-Ph+ cells at 3 mos; 2 were not in CCyR and with residual CD34+/lin-Ph+ cells at 3mos; 1 was in CCyR and with CD34+/lin-Ph- cells at 3 and 6 mos but with increasing qPCR. Only 1 pt with CD34+/lin-Ph- cells at all time points and with optimal molecular response harboured a NIL-resistant mutation at 26 mos of treatment.
None of the 22 pts (including 4 H-Sokal score pts = equal proportion of study cohort) in Molecular Response (MR) 3.0 at 3 mos had a positive FISH at 3 and 6 mos or failed treatment at follow-up.
Conclusion. Our final results on the whole cohort of pts confirm our preliminary data on the efficacy of NIL 300 g BID in early clearance of BM LSCs (CD34+/lin-Ph+) in newly diagnosed CP-CML patients tested at 3, 6 and 12 mos of treatment. Moreover, according to our data, fast disease debulking seems crucial for obtaining BM LSCs clearance and it can be speculated that the same mechanism responsible for this early MR 3.0 achievement is also capable of preventing H-Sokal risk pts from failing treatment.
Orlandi:Ariad: Honoraria; BMS: Honoraria; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal