Abstract
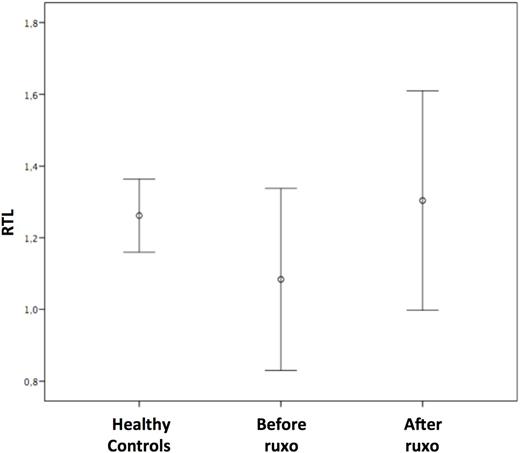
Introduction Telomeres are specialized structures of repetitive nucleotide sequences that cap the ends of human chromosomes able to maintain genome stability and protect the cell from progressive DNA shortening. Some recent reports describe reduced telomere length in myelofibrosis (MF) regardless of hydroxycarbamide therapy, suggesting a possible prognostic relevance for this biomarker. As yet, no studies have investigated telomere dynamics following treatment with ruxolitinib. Methods Eight primary MF and 3 post ET MF patients were given 15 mg or 20 mg of oral ruxolitinib twice daily (BID) depending on baseline platelet counts. The drug dose was escalated to 25mg BID in patients with an inadequate response and reduced when platelet counts dropped to <100,000/μL. Treatment was stopped when platelet levels fell below 50.000/μL. Telomere lengths were analyzed by quantitative PCR (q-PCR) as described by Cawthon (2002) and assessed before and after ruxolitinib at a median of 1000 days (range 113-1152). The relative telomere length (RTL) was determined as the telomere (T) to single copy gene (36B4) (S) ratio (T/S) normalized to a reference sample (K-562 DNA). Peripheral blood samples were also collected from 11 age and sex matched healthy controls. Results Median age at diagnosis was 72 years (range 53-83). The JAK2 V617F mutation was detected in 7 patients, while CALR and MPL were found in 2 and 1 patient, respectively. One patient was triple negative. All patients had splenomegaly with a median enlargement of 17 cm below the costal margin. Based on IPSS scores, 6 patients were assigned to the intermediate-2 risk category and 5 to the high risk category. Ruxolitinib was administered for a median of 1000 days (range 113-1152). Overall, patients received a median of 22 grams of ruxolitinib (range 4.6-44.5). Splenomegaly decreased by 60% (range 20-100%). Overall, BM fibrosis was improved in 35% of the cases and remained stable in 65%. Related samples Wilcoxon signed rank test performed before treatment with ruxolitinib, showed that mean RTL was shorter in patients compared to age and sex matched healthy controls (1.08 vs 1.26, respectively; p=0.09). After treatment, median RTL increased significantly (1.30 vs 1.08; p=0.018), showing overlapping values with the healthy controls. Median RTL elongation from baseline was 15% (Figure). Univariate and multivariate analyses included the following parameters: primary MF, presence of the JAK2 V617F mutation, high IPSS score, a decrease in splenomegaly of >50%, >50% bone marrow (BM) cellularity before and after treatment, pre and post treatment WHO scores for BM fibrosis of 3-4, duration of treatment >1000 days, total drug dose of >22 grams. Variables with a p-value lower than 0.2 in univariate analysis were included in multivariate analysis using a multi-step forward binary logistic regression model, where RLT >15% from baseline was considered a dependent variable. Only pre-treatment BM cellularity of >50% significantly correlated with >15% telomere elongation (p=0.004). Conclusion In our small cohort of patients, ruxolitinib proved capable of improving BM fibrosis and restoring telomere lengths to normal values. It is possible that ruxolitinib mediates modulation of the BM microenvironment, thereby stimulating stem cell hematopoiesis. It follows that peripheral blood telomere elongation could be indicative of the stem cellÕs ability to self-renew. This finding, if confirmed, would open a debate about the rationale of treatment with telomerase inhibitors in MF patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal