Abstract
Background: BT is an important public-health challenge in Italy. Seminal work has begun to better characterize disease prevalence, treatment patterns, and institutional capabilities within Italy.
Objective: Evaluation of current BT prevalence and treatment in Italy was done using proprietary IMS Health hospital audit data as the basis of a targeted survey of hematologists, pediatricians, and internists treating BT. We estimated the prevalence and distribution of patients with transfusion-dependent (TD) BT major (BT-M) and intermedia (BT-I), and non-transfusion-dependent BT (NTD BT), and characterized current treatment patterns.
Methods: A preliminary list of possible BT-treating centers was extracted from the IMS Health audit of hospitals and treatment centers across Italy, covering 656 public hospitals and accounting for 205,021 beds (85% of hospital capacity in Italy).The IMS Health hospital audit also included 95% of the pharmaceutical Direct Patient Distribution channel. This list was reviewed to identify centers receiving iron-chelating agents, thus identifying a total of 365 potentially BT-treating hospitals. One hematologist or pediatrician in each potential hospital ward was contacted by telephone. The physician was asked to provide details about the number of TD BT-M, TD BT-I, and NTD BT patients managed in their ward. In case of refusal to provide the required information, telephone or in-person interviews were organized with other specialists in the same facility. We identified 124 treatment centers, 114 of which were successfully surveyed (92% completed the survey). Subsequently we used a web-based questionnaire to ask a geographically stratified sample of 60 treating physicians about general treatment patterns, with a focus on potential drivers of health-care resource utilization. Responding physicians each referenced 3-4 records for patients currently under their care to inform their responses to our survey, and 205 records were referenced in total: 162 TD and 43 NTD patients.
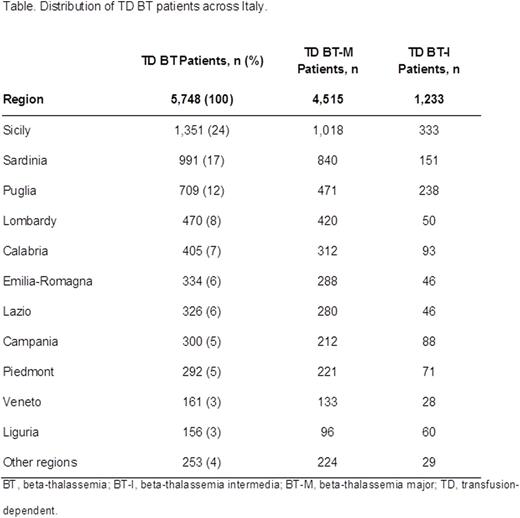
Results: In the 114 treatment centers surveyed, a total of 5,748 TD BT patients under regular treatment were reported, plus 1,296 additional patients receiving occasional treatment (NTD BT). Distribution of TD patients was heterogeneous, with the highest prevalence in Sicily, Sardinia, and Puglia. These regions each had > 500 TD patients and hosted in total 3,051 TD patients (Table). National patient volume was highly concentrated with the 7 largest centers managing 1,766 TD patients.
Based on the information in 205 patient records, complete blood count, ferritin level, echocardiography, and T2*-weighted MRI were the tests most commonly performed in the preceding 12 months. Of 162 TD patient records reviewed, 83 patients required 1-2 units of red blood cells per month, while 78 patients required ≥ 3 units per month (this information was not captured in 1 patient record). Deferasirox was the most commonly administered chelation treatment in TD patients, prescribed to 109 of 162 patients. Most TD patients (n = 126) had previously had a change of iron chelation treatment; 72 of these switched from deferoxamine to another agent. In-patient hospitalizations were estimated at 9 days/year, 7 days/year, and 4 days/year for TD BT-M, TD BT-I, and NTD BT patients, respectively. Endocrine pathologies were the most commonly reported comorbidity in all groups; 36 patients required medical treatment. Hepatitis C virus infection and hepatic and cardiac complications were also reported.
Conclusions: Prevalence of BT is regionally focused and likely to consume significant health-care resources for management of the disease and associated comorbidities. Although the telephone and web surveys used for our project were not designed to be a clinical trial, we were able to assess practices by a number of physicians (n = 60) for patients (n = 205) that were representative of the distribution of BT across Italy. We also screened the overall BT caseload of up to 365 potentially BT-treating centers in the country. Our survey complements recent work to understand the burden of this disease in Italy.
Angelucci:Novartis Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees. Burrows:Celgene Corporation: Research Funding; IMS Health s.r.l.: Employment. Losi:IMS Health: Employment; Celgene Corporation: Research Funding. Bartiromo:Celgene Corporation: Employment, Equity Ownership. Hu:Celgene Corporation: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal