Abstract
OBJECTIVES:
The prognosis for light chain amyloidosis (AL) is generally poor, particularly with cardiac, renal and multi-organ involvement. Treatment options for patients with relapsed/refractory light chain amyloidosis (RRAL) are limited. There is also a shortage of real-world evidence of treatment patterns and comparative effectiveness of treatment regimens for RRAL. The aims of this study were to describe treatment patterns in patients with newly diagnosed AL or RRAL, including types of treatment lines, as well as duration of treatment. Additionally, we aimed to characterize the incidence of vital organ (heart and kidney) deterioration and mortality following second-line or later-line treatment.
Methods:
This is a retrospective observational study of adult patients with AL using US Optum administrative claims data during 1/1/2008 to 6/30/2015. AL diagnosis was based on both the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) codes (277.30 or 277.39) and treatments with a claim for AL-specific anticancer systemic agents. AL patients who received stem cell transplantation (SCT) only without other AL-specific anticancer agent were not included. Patients who had claims for AL, in addition to another primary cancer or metastatic disease during the 12-month wash-out period were excluded. Patients receiving a second line of treatment for AL were classified as RRAL. Clinical outcomes included mortality and hospitalizations due to cardiac or renal deterioration. Health care resource utilization during one year following RRAL was reported by per patient per month.
RESULTS:
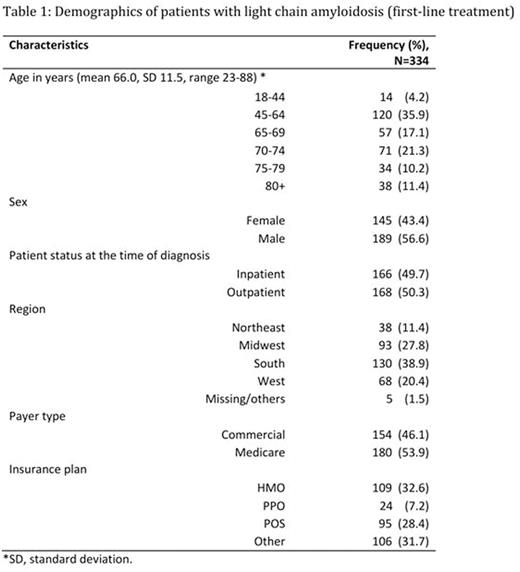
A total of 334 patients were identified with AL (Figure 1). The average age of AL patients was 66 years with a range of 23-88 years (Table 1). Based on prior therapies, 144 (43.1%) out of 334 patients were considered as RRAL. Majority (108/144) of the RRAL patients had evidence of cardiac or renal disease before the second line treatment.
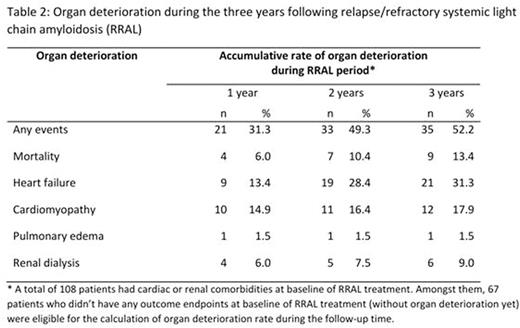
Over one third of the patients received monotherapy, and approximate half received doublet therapy for both first line AL and RRAL patients. Proteasome inhibitor (PI) based regimens were the most frequently used (41.0% for first-line AL, 30.6% for RRAL) followed by regimens based on immunomodulatory agents (15.0% for first-line AL, 28.5% for RRAL), alkylating agents (19.8% for first line AL, 14.6% for RRAL) and other combinations. SCT was part of the treatment regimen in 7.2% of first-line AL patients, and 12.5% of RRAL. Patients who received AL treatment with PI as first-line had higher reported occurrence of baseline (at initial diagnosis) heart failure (48.0% vs 30.9%), cardiomyopathy (41.4% vs 23.5%), and renal disease (57.6% vs 46.3%) compared to non-PI treated patients (p<0.05 for all). For patients who had cardiac or renal involvement, as shown in Table 2, 49.3% had organ deterioration during the two years following relapse, including mortality (10.4%), heart failure (28.4%), cardiomyopathy (16.4%), dialysis (7.5%) and pulmonary edema (1.5%). During the first year following relapse, the average healthcare resource utilization per patient per month included 0.16 inpatient admissions (0.07 due to cardiomyopathy/renal disease complications), 3.02 outpatient or office visits, 0.14 emergency room visits, and 0.05 intensive care unit admissions; and the average length of stay for inpatient admission was 8.2 days.
CONCLUSIONS:
In this real-world cohort study of patients with AL, majority of patients had cardiac or renal involvement at the start of RRAL treatment. PI regimens are the most common treatment in both 1st line and 2nd line. PI-based regimens were chosen in patients with worse clinical characteristics prior to the treatment, likely because of the perceived need in these patients for the rapid and deep free light chain responses which can be generated by PI-based therapies. Clinical outcomes remained poor emphasizing the need for medications to improve care for patients with RRAL.
Lin:Takeda: Employment. Asche:Takeda: Consultancy. Ren:Takeda: Consultancy. Yong:Takeda: Employment. Parameswaran:Takeda: Consultancy. Luptakova:Takeda Oncology: Employment. Faller:Takeda: Employment. Seal:Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal