Abstract
INTRODUCTION
Novex is a rituximab biosimilar approved in Argentina. From January 2015 to June 2016 as part of the Risk Management Plan approved by ANMAT, every treatment detected was recorded and a follow up was implemented in order to obtain safety information.
The aim of this communication is to present the information gathered during this pharmacovigilance program.
MATERIALS AND METHODS
All treatments with at least 1 follow up point were selected and reviewed.
Treatment duration until last follow up checkpoint or date of treatment finalization was recorded.
We summarized demographic data (age, gender), indication distribution, treatment exposition (duration expressed in days and number of cycles received), adverse event frequency (cumulative incidence and incidence density) and distribution.
RESULTS
We collected 345 treatment notifications with at least 1 follow up point.
Gender distribution: 53.2 % of patients were male patients. Age range was 10 to 90 years old, with a mean of 63.9 years.
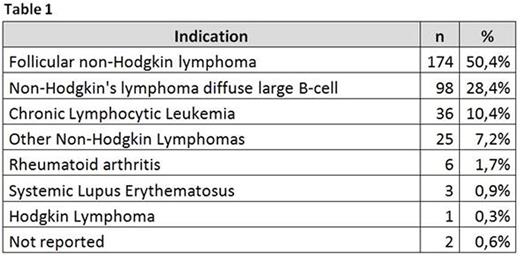
The indications distribution is summarized in table 1. More than 90% of them were hematological.
Average treatment duration was of 235 days with a median duration of 210 days (IQR = 152 - 311).
Mean number of cycles 5.5; range (1-12).During the reported period a total of 12 Individual Case Safety Reports (ICSR) were received. This represents a cumulative incidence of 3.5 %, an incidence density of 0.015 ICSR notifications per 100 days of treatment and 0.99 per 100 administered cycles.
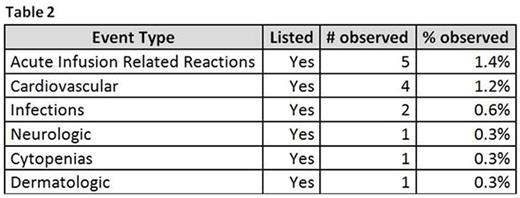
These 12 ICSR totalized 14 different diagnoses. Of them, the most frequent was Acute Infusion Related Reaction (AIRR), as expected.
All AE but 1 were classified as serious (SAE) because they had at least 1 manifestation that prolonged hospitalization or endangered life.
As expected, the most frequent AE notified was AIRR (5 cases), followed by cardiovascular manifestations (2 arrhythmia, 1 cardiac failure and 1 ischemic stroke), infections (1 pneumonia, 1 progressive multifocal leukoencephalopathy), neurologic (1 paresthesia), cytopenias (1 pancytopenia) and cutaneous (1 bullous dermatitis).
Most of the acute manifestations occurred within the 2 initial infusions of Rituximab, as described.
CONCLUSION
The observed AE frequency and distribution was consistent with product specifications.
No AE notified were unlisted or were notified with greater frequency than expected.
Treatments registry resulted in a suitable way to detect AE that complements spontaneous reporting.
Fernandez:Laboratorio ELEA S.A.C.I.F y A: Employment. Penna:Laboratorio ELEA S.A.C.I.F y A: Employment. Gomez:Laboratorio ELEA S.A.C.I.F y A: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal