Abstract
Introduction: Light chain (AL) amyloidosis is a rare disease characterized by misfolded amyloid protein deposits in tissues and vital organs which may lead to organ failure, disability, and death. Little is known about the burden of AL amyloidosis on health-related quality of life (HRQoL) - particularly whether patients in different types of studies experience the same degree and type of burden or whether there are unique differences.The objective of this study is to compare the HRQoL profile of patients with AL amyloidosis from clinic-, community-, and clinical trial-based samples to a general population (GP) sample and other benchmarks from more commonly known diseases.
Methods
Sample
Data were drawn from three different samples of AL amyloidosis patients:
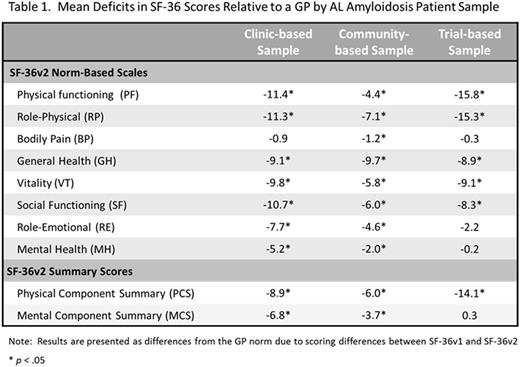
1. A clinic-based sample of AL amyloidosis patients seen at the Amyloidosis Center at Boston University between 1994 and 2014 who completed the HRQoL survey within 30 days of their initial evaluation (n=1,438)
2. A community-based sample of patients who completed a cross-sectional online survey in the Fall of 2015 (n=341)
3. A trial-based sample of treatment naïve patients with cardiac involvement who completed the HRQoL survey during baseline data collection of the VITAL Amyloidosis Study, a Phase 3 randomized control trial (RCT) of NEOD001 (clinical trials.gov ID: NCT02312206; n=85)
Measures
All three studies assessed HRQoL using the SF-36® Health Survey (SF-36). The SF-36v1 was administered to the clinic-based sample and the SF-36v2 was administered to the community- and trial-based samples. The SF-36 measures eight domain scales (physical functioning (PF), role limitations due to physical health problems (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role limitations due to emotional health problems (RE), and mental health (MH)) and two component summary measures: physical (PCS) and mental (MCS).
Analysis
Analysis of variance was used to compare the norm-based SF-36 scores from patients with AL amyloidosis to that from samples representing a GP norm. The GP data were adjusted to the age and gender distribution of the patient samples using separate ordinary least-squares regression models, with each SF-36 scale or summary score as a dependent variable. Using the same method, mean SF-36 scales and summary scores in the three AL amyloidosis patient samples were compared to the mean SF-36 scores in other disease populations, including congestive heart failure (CHF) and kidney disease (KD). Established minimally important differences (MIDs) were used to determine whether deficits in HRQoL were clinically meaningful, as well as statistically significant.
Results: Differences between AL amyloidosis patients' SF-36 scores and those of the GP are presented in Table 1 by sample. Relative to the GP, patients with AL amyloidosis exhibited broad deficits in HRQoL, particularly related to physical well-being. Deficits were more prominent for PCS where the differences between the GP scores and the scores observed in all three AL amyloidosis samples were both statistically significant and clinically meaningful (p < 0.001 and D ≥ 2.0 points for all).
Differing patterns of relative burden emerged when AL amyloidosis samples were compared to other common disease benchmarks:
· As compared to the CHF benchmarks, scores for PCS among the community-based sample were statistically and clinically better (p < 0.001 and D=4.5); whereas, PCS scores were significantly worse among the trial-based sample (p < 0.01 and D=-8.9).
· Relative to KD benchmarks, the trial-based, treatment naïve sample reported significant deficits across many scores that were comparable in the community-based sample, including RP, VT, and PCS.
Conclusions: Regardless of the data source, patients with AL amyloidosis exhibited significant and clinically meaningful deficits in HRQoL compared to the GP. Despite heterogeneity across the three samples in terms of duration and severity of disease, deficits related to physical well-being were consistent and clinically meaningful. Different patterns of deficits emerged when comparing the three samples to other disease benchmarks. This may be due to the heterogeneity in the community-based sample where patients varied in duration of disease and course of treatment versus the other AL amyloidosis samples which were comprised of newly diagnosed and/or treatment naïve patients.
Bayliss:Prothena Biosciences Inc: Research Funding. White:Prothena Biosciences Inc: Research Funding. McCausland:Prothena Biosciences Inc: Research Funding. Guthrie:Prothena: Employment, Equity Ownership, Other: Leadership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal