Abstract
Background:
Patients with hematological malignancies are at an increased risk for developing both primary and recurrent Clostridium difficile Infection (RCDI) along with complications such as toxic mega colon and treatment failure likely due to underlying immunosuppression and frequent use of broad spectrum antibiotics that lead to altered gut microbiome. Fecal Microbiota Transplantation (FMT) is an effective treatment for RCDI (Brandt et. al; 2012). However, experience in patients with hematologic malignancies is sparse and most clinical trials exclude these patients due to potential complications. We report the largest case series to date from a single institution evaluating the safety and efficacy of FMT for RCDI in patients with hematologic malignancies.
Methods:
After IRB approval, a database of 452 RCDI patients treated with FMT between August 2012 and June 2016 was reviewed to identify those with an underlying hematologic malignancy. Data regarding demographics, hematologic disease, C. difficile history, treatments, and outcomes were retrospectively abstracted from the electronic medical record.
Results:
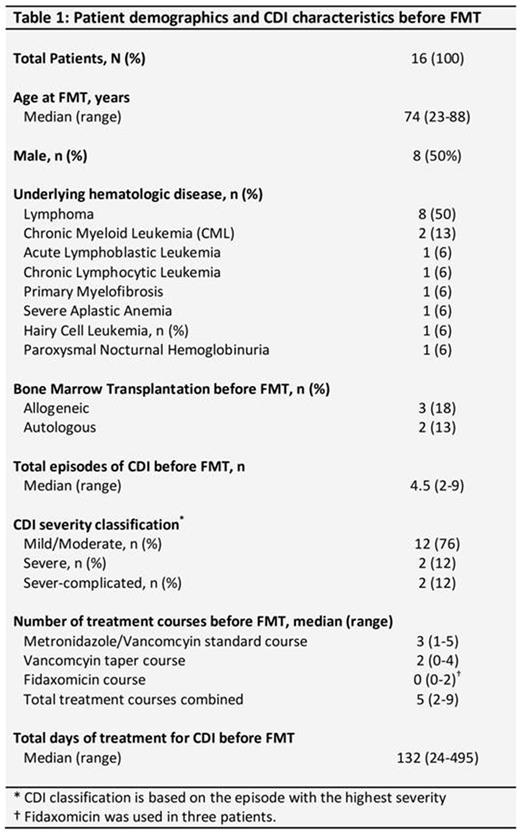
Sixteen patients (median age 74 years; male 50%) with known hematologic malignancies underwent FMT during the study period. The underlying diagnoses are outlined in Table 1. Five patients had received hematopoietic cell transplantation (3 allogeneic and 2 autologous) prior to FMT. Patients had a median of 4.5 (range 2-9) CDI episodes before FMT, and 4 of them had severe/severe-complicated CDI at some stage. Prior treatments included a median of 3 (range 1-5) standard vancomycin/metronidazole courses, median of 2 (range 0-4) vancomycin taper courses, fidaxomicin in 3 patients, and chronic vancomycin suppression in one patient. Diarrheal symptoms were in remission in all but 3 patients in the week before FMT.
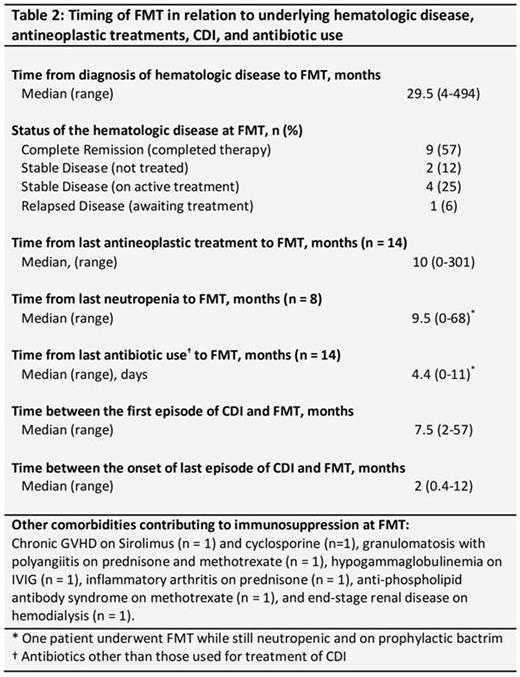
FMT was performed via colonoscopy in all patients. At the time of FMT, hematologic malignancies were in complete remission in 9 patients, stable on active treatment in 4, stable off treatment in 2, and relapsed awaiting treatment in 1 patient. Median time from last anti-neoplastic treatment (n = 14) and from last neutropenia (n = 8) to FMT were 10 (range 0-301) and 9.5 (0-68) months, respectively. One patient with hairy cell leukemia was still neutropenic and on prophylactic oral trimethoprim/sulfamethoxazole at the time of FMT. Five patients were on active immunosuppressive medications, including prednisone (n = 2), methotrexate (n = 2), sirolimus (n =1), and cyclosporine (n =1) for related comorbidities at the time of FMT (Table 2).
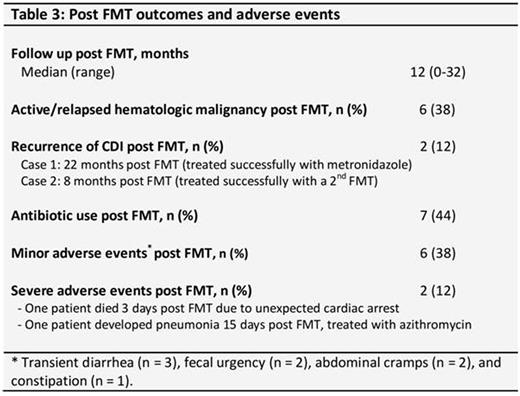
At last follow up (median 12, range 0-32 months), 6 patients had active/relapsed hematological disease, 6 had received additional antineoplastic treatments, and 7 had received additional antibiotics. RCDI developed in two (12%) patients at 8 and 22 months post FMT secondary to exposure to broad spectrum antimicrobials. These patients were successfully treated with a second FMT and with metronidazole, respectively. Severe adverse events included death in one patient that occurred 3 days post FMT due to unexpected cardiac arrest and was deemed unrelated to the procedure. Another patient developed community-acquired pneumonia 15 days post FMT and was treated successfully with oral azithromycin. Minor adverse events within the first two weeks post FMT were noted in 6 (38%) patients (self-limited diarrhea in 3, fecal urgency in 2, abdominal cramps in 2, and constipation in one patient) (Table 3). Only one patient had persistent diarrhea shortly after FMT, with the cause attributed to underlying Crohn's disease. No complications related to the colonoscopy procedure were noted.
Conclusion:
FMT appears to be a safe and effective therapeutic option for RCDI in patients with hematological malignancies. Considering very few adverse events and particularly no infectious complications in our series, we conclude that immunosuppression should not preclude the use of FMT for treatment of RCDI in this high risk population. These results need prospective validation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal