Abstract
Background: Before SWOG S0777 (Durie BGM at al. ASH 2015, abstract #25) it was not clear whether standard upfront regimens VRd (bortezomib, lenalidomide, dexamethasone) or Rd (lenalidomide, dexamethasone) yield different survival outcomes for newly diagnosed myeloma patients (pts.) but it was known that the higher response rate of VRd comes at a higher risk for severe peripheral neuropathy. Since no test predicts who requires the more toxic regimen to avoid adverse myeloma effects we designed a carepath that tailors therapy according to early response endpoints.
Methods: Newly diagnosed symptomatic MM patients (pts) with measurable disease who were not eligible for or elected against a clinical trial were advised to begin a 2-drug regimen of lenalidomide (R) and weekly dexamethasone (d) or, if cast nephropathy suspected or R copay too high, bortezomib (V) and dexamethasone (D). Depending on response assessment per IMWG 2011 criteria after each cycle, treatment intensity was to be increased with sequential addition of agents (R or V, cyclophosphamide, and then liposomal doxorubicin) when there was not at least minor response (MR) after the first, or at least partial response (PR) after the second cycle of an administered combination. Once PR was reached, pts. were evaluated for high dose melphalan (HDM) and ASCT or consolidation with their induction regimen followed in either case by lenalidomide or bortezomib maintenance. After IRB approval, pts treated on carepath were identified through our registry and electronic medical records were reviewed.
Results: From Oct 2012 to Dec 2015 the carepath was used in 91 pts. Their median age at treatment start was 64 years (34-84), 40 (44%) were ≥ 65, 11 (12%) ≥ 75 years old; at least one cytogenetic risk study (MyPRS®, FISH panel, karyotype analysis) was obtained in 81 (89%) and of them 23 (28.4%) had high risk features (MyPRS® score > 45.2, del17p, 1q amp, t4;14, t;14;16 and non-hyperdiploid karyotype abnormalities), 33 (36%) had ISS stage III and 21 (23%) had serum creatinine ≥ 2mg/dL, 6 (7%) were on dialysis. At median follow up of 20.5 months (1.7-44.6), at least PR was achieved in 84 pts (92%), at least very good partial remission (VGPR) in 63 (69%), with negative immunofixation in blood and urine in 24 (26%) and complete remission (CR) documented by bone marrow exam in 6 (7%). Only one pt (1%) had progressive disease as best response, stable disease and MR were seen in 3 pts. (3%) each. Induction required 2, 3, 4 and 5 drugs in 49 (54%), 33 (36%), 8 (9%), 1 (1%) pts, respectively, and was followed by high dose melphalan and autologous stem cell transplant in 19 (21%). Sixty-three pts. (69%) remain on carepath treatment without progression, while 28 (31%) have experienced progression with skeletal events in 4 (4%) and ARF in 2 pts. (2%). Nine pts. (10%) have died, 4 while in remission of whom 3 suffered a bleed while anticoagulated for DVT or pre-existing A-fib and died 1 after transition to hospice; the remaining 5 died after progression, of infection (3), secondary plasma cell leukemia (1), or after transition to hospice (2). No patient developed shingles or second primary malignancies so far; mild peripheral neuropathy (PNP) occurred in 27 pts (30%), severe or painful PNP in 4 (4%) and 8 pts. (9%) suffered venous thromboembolism (9%) on DVT prophylaxis with aspirin.
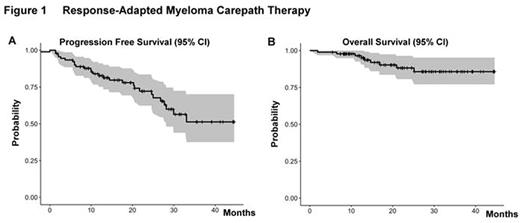
Conclusions: Carepath therapy required only two drugs during induction in over 50% of patients to achieve an overall response rate of 92% with ≥ VGPR in 69% in a real-world setting without exclusion of patients on dialysis. Reduction in costs and side effects compared to upfront use of VRd for everyone were accompanied by promising early Kaplan-Meier estimates for progression-free and overall survival (Fig. 1) supporting the carepath principle and future randomized comparison of response-adapted therapy to fixed regimens like the new SWOG S0777 defined standard triplet VRd.
Reu:Novartis: Research Funding; Takeda: Research Funding; Celgene: Research Funding; Signal Genetics: Consultancy. Valent:Celgene: Speakers Bureau; Takeda: Speakers Bureau. Faiman:Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy; Takeda: Consultancy. Hamilton:Takeda: Speakers Bureau. Smith:Celgene: Honoraria; Spectrum: Honoraria; Genentech: Honoraria; Abbvie: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal