Abstract
Introduction: The mechanisms involved in cholestatic liver injury remain elusive although emerging evidence indicate neutrophils, rather than bile acids (BAs), are the predominant source of damage. Neutrophil extracellular traps (NETs) are double-edge swords that serve to ensnare and kill microbial pathogens but also contribute to excessive inflammation and tissue damage. However, the role of NETs during bile duct ligation (BDL)-induced cholestatic liver injury is largely unknown. Moreover, whether neutrophils and BAs cooperatively participate in this process also needs to be investigated. Our objectives were to localize and determine the magnitude of NETs in the hepatic microvasculature, define the role of NETs on hematological and histological changes, and characterize the role of BAs in the induction of NETs in BDL mice.
Methods: BDL was induced in C57BL/6 mice by ligation of the common bile duct. After 3 days, liver tissue was collected and NETs were detected by immunostaining and western blot. Serum myeloperoxidase (MPO)-DNA complexes were measured by ELISA. Cholestatic liver injury was characterized by hematological and histological assessment. NETs were dismantled by DNase I or depleted with peptidylarginine deiminase 4 (PAD4) inhibitor Cl-amidine. The apoptosis of hepatocytes was measured by TUNEL and the induction of chemokines on Kupffer cells was analyzed by quantitative polymerase chain reaction (qPCR). BAs and inflammatory cytokines were utilized collaboratively to induce NETs formation both in vitro and in vivo. Diphenylene iodonium (DPI) was used to inhibit reactive oxygen species (ROS) formation. Plasma was obtained from healthy controls and patients with entrahepatic cholestasis.
Results: We found that NETs formed in the sinusoids of cholestatic liver lobes in vivo, which was associated with significantly increased serum MPO-DNA complexes (P<0.01) and tissue levels of citrullinated-histone 3 (P<0.05) in BDL mice compared with sham operated mice. The formation of NETs exacerbated BDL-induced liver injury as evidenced by markedly increased levels of serum alamine transaminase (ALT) and percentage of necrotic tissue area (both P<0.05 vs. sham group, Figure 1). Depleting of NETs with DNase I or Cl-amidine decreased NETs formation, protected hepatocytes and dramatically alleviated cholestatic liver injury, indicating the pathophysiological role of NETs in BDL mice (Figure 1). In addition, NETs promoted the apoptosis of hepatocytes and induced the release of chemokines from Kupffer cells in BDL mice compared to sham group. Both effects could be inhibited by DNase I or Cl-amidine. Moreover, BAs induced mouse neutrophils, primed with inflammatory cytokines, to release NETs in vitro in a dose-dependent manner and mediated by PAD4 and ROS. Intravenously injection of BAs and inflammatory cytokines to healthy mice lead to the formation of NETs and exacerbated liver injury, suggesting that depleting NETs can prevent organ damage. Furthermore, we found NETs markers were significantly increased in the serum of cholestatic patients, correlating positively with increased levels of ALT and BAs.
Conclusions: Our results suggest that BAs promote NETs formation via PAD4 and ROS. Development of NETs subsequently initiates inflammatory responses and exacerbates organ damage during BDL-induced cholestatic liver injury, and may therefore serve as a promising therapeutic target in cholestasis.
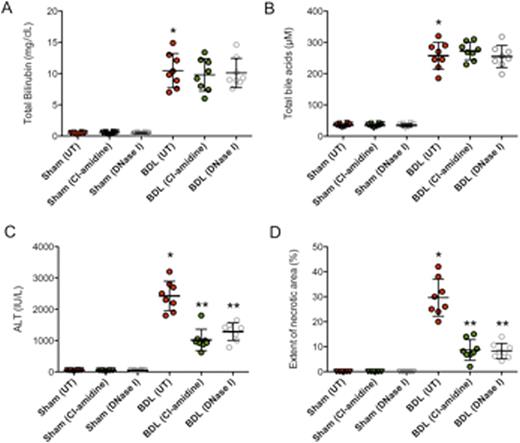
Inhibition of NET formation by PAD4 inhibitor (Cl-amidine) or DNase I protects cholestatic liver injury in BDL mice. Serum total bilirubin (A), total bile acids (B), and ALT levels (C) were assessed in control-, Cl-amidine-, or DNase I treated mice after either 3 days of sham laparotomy or BDL. (D) Quantification of necrotic hepatocytes in H&E stained liver sections from control-, Cl-amidine-, or DNase I treated mice 3 days after sham laparotomy or BDL. *P < 0.05 untreated BDL mice vs. sham mice. **P < 0.05 Cl-amidine or DNase I treated BDL mice vs. untreated BDL mice.
Inhibition of NET formation by PAD4 inhibitor (Cl-amidine) or DNase I protects cholestatic liver injury in BDL mice. Serum total bilirubin (A), total bile acids (B), and ALT levels (C) were assessed in control-, Cl-amidine-, or DNase I treated mice after either 3 days of sham laparotomy or BDL. (D) Quantification of necrotic hepatocytes in H&E stained liver sections from control-, Cl-amidine-, or DNase I treated mice 3 days after sham laparotomy or BDL. *P < 0.05 untreated BDL mice vs. sham mice. **P < 0.05 Cl-amidine or DNase I treated BDL mice vs. untreated BDL mice.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal