Abstract
Introduction. In France as in many other countries, first-line treatment of adult immune thrombocytopenia (ITP) is based on conventional dose (1 mg/kg/d) oral steroids (CDOS) during 3 weeks. Intravenous polyvalent immunoglobulin (IVIg) is added in case of severe bleeding. However, conventional dose (1 mg/kg/d) intravenous methylprednisolone (CDMP) is often used as a first-line treatment during a few days before continuation with oral prednisone. Indeed, CDMP may induce earlier response in comparison with CDOS due to the absence of intestinal absorption and of hepatic metabolism to be converted in the active substance. No study has assessed the benefits and the safety of this strategy in comparison with CDOS. This was the aim of this study.
Methods. This study was conducted within the CARMEN registry. It is the cohort of all newly diagnosed ITP adults (≥ 18 years) recorded in the Midi-Pyrénées region, South of France, from June 2013 (still ongoing). For the present study, we selected all ITP adults included in the CARMEN registry between 05/01/2013 and 12/31/2015 with platelet count <30 x 109/L and treated with corticosteroids. All patients were hospitalized at treatment initiation. We compared two groups: the patients treated by CDOS since ITP diagnosis (CDOS group) and those treated primarily by CDMP followed by CDOS (CDMP group). The primary outcome was the time to response, defined as the time between treatment initiation and response (no bleeding and platelet count >30 x 109/L twice 7 days apart). Secondary outcomes were: the time to complete response, defined as the time between treatment initiation and complete response (no bleeding and platelet count >100 x 109/L twice 7 days apart); the rates of response and of complete response; and the occurrence of adverse drug reactions (ADRs) due to corticosteroids, analyzed by a pharmacologist and an internal medicine physician using the WHO causality scale. The primary outcome and the time to complete response were assessed using Kaplan-Meier curves. The index date was the date of start of treatment. Follow-up was censored in case of occurrence of the outcome, date of start of second-line treatment, death or date of last follow-up, whichever occurred first. All analyzes were adjusted using a propensity score (PS) assessing the probability of being exposed to CDMP vs. CDOS as well as for concurrent IVIg use. The PS included the following variables: age, gender, comorbidity Charlson score, bleeding score, platelet count before treatment and secondary vs. primary ITP. Log-rank tests were used to compare the 2 groups. Hazard ratios (HRs) were calculated using Cox models.
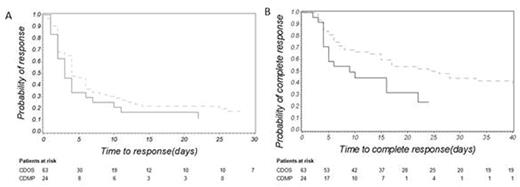
Results. The study included 87 patients (24 in the CDMP group and 63 in the CDOS group). Mean age was 67 years (± 22) and 48% were females. ITP was primary in 84% of patients. Mean platelet count was 9.7 x 109/L (± 8.2). Thirty-four patients were also exposed to IVIg. CDMP was prescribed for 3 days in median (range: 1-10). Mean follow-up was 9.4 months (± 8.5). The overall response and complete response rates were 88.5% and 67%, respectively. The median time to response was 3 days in the CDMP group and 4 days in the CDOS group. It was 6.5 and 17 days for complete response, respectively. Response and complete response occurred earlier in the CDMP group (Figure 1A and 1B). However, only a statistical trend was observed for the time to response (adjusted HR: 1.35, 95% confidence interval - CI: 0.76 - 2.41, p<0.3). The adjusted HR for complete response was 2.29 (95% CI: 1.20 - 4.36, p<0.02). Response rates were 87.5% in the CDMP group and 89.0% in the CDOS group (p=1). Complete response rates were 71.0% and 65.0%, respectively (p=0.6). ADRs occurred in 29 (33.3%) patients who experienced 37 corticosteroid-related ADRs. These were mainly infections and diabetes mellitus. There was no difference between the 2 groups.
Conclusions. CDMP resulted in an earlier response and complete response than CDOS. Only a trend was observed for the time to response probably due to lack of power to detect smaller variations in platelet counts. In hospitalized patients at ITP onset, initiating CDMP may reduce hospital stay duration.
A. Kaplan-Meier curve assessing the probability of response in the CDMP group (full line) and the CDOS group (dotted line); log-rank test: p<0.3. B. Kaplan-Meier curve assessing the probability of complete response in the CDMP group (full line) and the CDOS group (dotted line); log-rank test: p<0.0005.
A. Kaplan-Meier curve assessing the probability of response in the CDMP group (full line) and the CDOS group (dotted line); log-rank test: p<0.3. B. Kaplan-Meier curve assessing the probability of complete response in the CDMP group (full line) and the CDOS group (dotted line); log-rank test: p<0.0005.
Beyne-Rauzy:Celgene, Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal