Abstract
Introduction: Early identification of coagulopathy has crucial clinical implications for the management of patients who are critically ill, severely injured or who are on anticoagulation therapy. Conventional laboratory-based coagulation tests are time-consuming, labor-intensive and costly. Currently available point-of-care (POC) devices are intended for use in specific patient populations (warfarin) or measurements are insensitive due to interference from the device surface. There is a growing need for a low-cost, easy-to-use, portable platform for POC assessment of the complete hemostatic process outside of the laboratory setting.
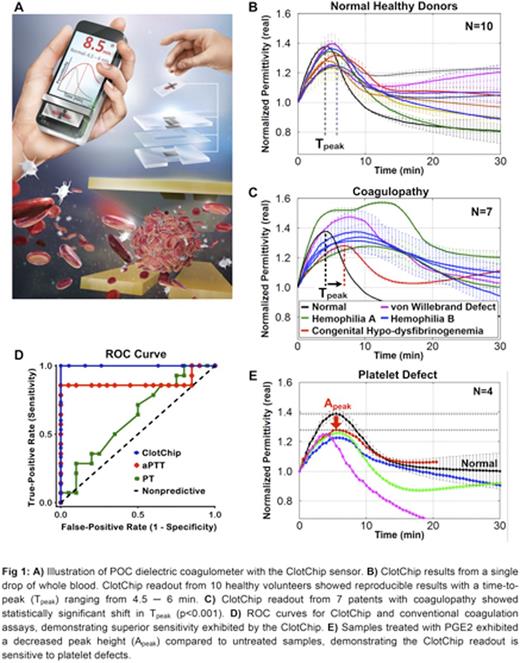
Methods & Results: We have developed a novel dielectric microsensor, termed ClotChip, that is based on the fully electrical technique of dielectric spectroscopy (DS) and is sensitive to multiple coagulation factors and platelet activity, thereby allowing comprehensive blood coagulation assessment in a miniaturized, portable measurement platform (Fig 1A). ClotChip features a three-dimensional, parallel-plate, capacitive sensing structure integrated into a low-cost (material cost <$1) and disposable microfluidic biochip with miniscule sample volume (<10µL). The sensor is constructed from biocompatible and chemically inert materials (polymethyl methacrylate substrate and gold electrodes) to minimize the potential for artificial contact activation. ClotChip measurements were performed with whole blood from healthy volunteers (n=10) collected in 3.2% sodium citrate. Coagulation was induced with CaCl2. The ClotChip curves exhibited a reproducible rise to peak within 4.5 to 6 min (Tpeak; Fig 1B). Conventional coagulation tests were also performed in each of the healthy samples in duplicate and confirmed normal aPTT and PT values. ClotChip measurements were then performed in 7 clinical samples obtained from patients with coagulopathy. These patients were referred to a specialized Hematology clinic for work-up of coagulopathy. Four patients suffered from intrinsic pathway defects (2 with Hemophilia A, 3 with Hemophilia B), one patient from acquired von Willebrand (vW) factor defect and one patient from mild congenital hypo-dysfibrinogenemia (Fig 1C). Compared to the normal curve, all samples from patients with coagulopathy exhibited an abnormal curve with an extended Tpeak range of 7 to 15 minutes (p = 0.0004). An ROC curve was generated. The true positive rate was plotted against the false positive rate in Fig 1D. The Area Under the Curve for ClotChip (1.00) was higher than that of both aPTT (0.7813) and PT (0.5859), illustrating that ClotChip Tpeak parameter has superior sensitivity compared to conventional screening coagulation tests. Next, ClotChip measurements were performed with whole blood from 4 healthy donors after the samples were treated with 1µM prostaglandin E2 (PGE2) to inhibit platelet aggregation. We determined that PGE2-treated samples exhibited a statistically significant (p=0.03) lower peak height (Apeak) than that of untreated samples (Fig 1E) while Tpeak values remained unchanged between treated and untreated samples. This shows that the Apeak parameter is sensitive in response to platelet function and thatClotChip is able to detect platelet function defects.
Conclusions: We have developed a novel dielectric microfluidic sensor (ClotChip) that is sensitive to multiple coagulation factors and platelet activity, thereby allowing whole blood assessment of hemostasis in a single disposable sensor. TheClotChip will bring blood coagulation testing closer to the patient for time-sensitive applications such as diagnosis of the bleeding patient and in trauma-induced coagulopathy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal