Abstract
BACKGROUND: Chronic transfusion is associated with an increased risk of bone marrow graft rejection. In prior studies with mouse models, minor histocompatibility antigens in red blood cell (RBC) and platelet products were presented in the context of recipient MHC class I to prime recipient cytotoxic T cells; these represent cross presentation and cross priming, respectively. Once primed in the recipient, these T cells may attack allogeneic bone marrow grafts. Thus, understanding the mechanism of transfusion-induced T cell cross priming may lead to new methods to reduce bone marrow graft rejection in chronically transfused patients. Cross presentation is performed by a specialized subset of antigen presenting cells (APCs) - CD11b lo, XCR1+ dendritic cells (XCR DCs). Transfusion of RBCs after prolonged refrigerated storage induces erythrophagocytosis and pro-inflammatory gene expression in the spleen. Fluorescent tracking of transfused RBCs showed that splenic red pulp macrophages (RPMs) ingest the majority of damaged RBCs, but RPMs are weak APCs. Splenic dendritic cells, including XCR DCs, also display increased uptake of stored vs. fresh RBCs. These data suggested that refrigerated storage may increase cross presentation of RBC antigens, thereby enhancing T cell cross priming.
AIMS: To compare T cell cross priming after transfusion of fresh and refrigerator-stored RBCs, a mouse model was used with transgenic OVA-carrying RBCs as the antigen source and transgenic naive MHC class I-restricted (H-2Kb) OVA-specific T cells (OT-1) as responders. In parallel, an in-vitro system was established to determine the cellular elements required for cross presentation of RBC antigens.
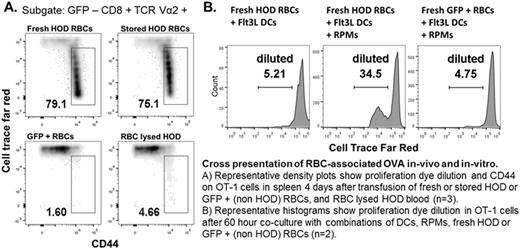
METHODS: Purified CD8 T cells from OT-1 mice were loaded with the cell proliferation tracking dye Cell Trace Far Red (CTFR) and adoptively transferred (4 x 10e6 per mouse) into cohorts of 8-12 week old C57BL/6 GFP+ mice (n=3 per group). The next day mice were transfused with 400 uL of fresh (<24 hours old), or stored RBCs (14 days old) from transgenic HOD mice (RBCs express surface HEL, OVA, and Duffy antigens). After 96 hours, flow cytometry was used to assess OT-1 cell proliferation by dye dilution and the expression of activation markers CD44, CD122 and CD62L. Control transfusions with non-HOD GFP+ RBCs confirmed the antigen specificity of the response, and transfusions of HOD blood that had undergone RBC lysis ruled out direct antigen presentation by MHC identical white cells in the transfusate. For the in-vitro cross presentation assay, 1x10e5 CTFR-loaded OT-1 cells were plated in U-bottom 96 well plates with all possible combinations of fresh or stored HOD RBCs (50 x 10e6 per well), bone marrow Flt3L-derived C57BL/6 murine dendritic cells (Flt3L-DCs, 5 X 10e4 per well), and magnetically selected BALB/c (H-2Kd) RPMs (5 x 10e4 per well). MHC mismatch rules out antigen presentation by BALB/c RPMs (H-2Kd) to OT-I T cells (H-2Kb).
RESULTS: Transfusion of both fresh and stored HOD RBCs induced vigorous proliferation and activation of OT-1 cells. After 4 days, no differences were seen in the proliferation and activation profiles of OT-1 cells in mice receiving fresh (78±4% CD44hi, CTFR diluted) vs. stored (79±5%) HOD RBCs. The in-vitro cross presentation assay showed weak to absent OVA-specific OT-1 proliferation with co-cultures including fresh or stored HOD RBCs alone, and fresh or stored HOD RBCs plus Flt3L-DCs. In contrast, cultures including RPMs and Flt3L-DCs showed enhanced OVA-specific OT-1 proliferation with fresh (29±10% CTFR diluted) and stored (25±2%) HOD RBCs.
CONCLUSION: Cross priming by fresh RBCs was stronger than expected based on prior studies showing minimal RBC uptake by XCR DCs after fresh GFP + RBC transfusion. Cross priming in this system may be more sensitive due to the high frequency of antigen specific T cells and, thus, is saturated by the small antigen load delivered by fresh transfusion. It is also possible that direct uptake of RBCs by DCs harms the cross-presentation machinery and offsets the effect of increased RBC antigen delivery. Optimal cross-presentation of RBC antigen may require cooperation with cells functionally specialized for metabolizing RBC by-products. Indeed, the in-vitro study shows RPMs promote DC-mediated cross presentation of antigens on fresh and stored RBCs. Thus, RPMs may share ingested RBC antigen with adjacent DCs and increase DC activation by secreting inflammatory cytokines.
.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal