Abstract
C5-blockade with eculizumab prevents complement-mediated intravascular hemolysis in PNH patients and its clinical consequences. However, a distinct population of PNH red blood cells bound with C3 fragments appears in almost all treated patients. This C3 binding results in extravascular hemolysis that in some patients reduces the clinical benefit from eculizumab. In each PNH patients on eculizumab there are always two distinct populations of PNH red blood cells, one with (C3+) and one without (C3-) C3 binding. This phenomenon is somehow paradoxical since the glycosylphosphatidylinositol (GPI)-linked complement regulators, CD55 and CD59, are uniformly deficient on the surface of PNH red cells.
To investigate this phenomenon, we have modeled in vitro the C3 binding in the context of C5 blockade by incubating red blood cells from PNH patients with AB0-matched sera from patients on eculizumab. Complement alternative pathway has been activated by mild acidification (in presence of Mg/EGTA to prevent the activation of complement classical pathway) and C3 binding has been assessed by flow cytometry at serial time points. In these experimental conditions a fraction of PNH red blood cells, similar to what happens in vivo, become promptly C3+ and its size increases with the time: from 9.4±2.7% after 5 minutes to 21.2±9.5% after 24 hours.
The membrane defects of PNH cells suggested that the deficiency of CD55, which regulates the formation and accelerates the dissociation of C3 convertases, should be responsible for C3 binding to PNH red blood cells in presence of eculizumab (Parker CJ. Hematology Am Soc Hematol Educ Program. 2011;2011:21-29).
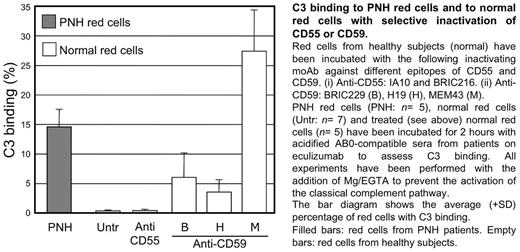
In order to verify experimentally this hypothesis we have inactivated CD55 or CD59 on normal red blood cells by using blocking monoclonal antibodies (moAb - listed in the figure legend), and we have tested them in vitro upon activation of complement alternative pathway by mild acidification in presence or absence of C5 blockade.
We found that CD55 inactivation on normal red blood cells results neither in hemolysis (without C5 blockade) nor in any C3 binding (with C5 blockade). As expected without C5 blockade CD59-inactivated normal red blood cells undergo hemolysis but, surprisingly, we found that in presence of C5 blockade they become bound with C3 fragments (Figure 1), just as it occurs in vivo in PNH patients on eculizumab. The simultaneous inactivation of both CD55 and CD59 further increased the level of C3 binding. Thus, at variance with the starting hypothesis, the deficiency of CD59, not that of CD55, plays the major role in C3 binding to PNH red cells of patients on eculizumab.
Therapeutic C5 blockade in PNH patients has unmasked a novel function of CD59: in addition to prevent MAC formation, it plays a central role also in the regulation of C3 activation on cell surface through molecular mechanisms not elucidated yet. It remains to be established the physiological role, if any, of this novel function of CD59 and whether it play a role in determining the pleomorphic clinical features of the congenital CD59 deficiency. Finally, these findings may lead to investigate innovative approaches to reduce C3 binding and extravascular hemolysis in PNH patients on eculizumab and, in a broader context, to modulate complement activity.
Risitano:Novartis: Research Funding; Alexion Pharmaceuticals: Other: lecture fees, Research Funding; Rapharma: Research Funding; Alnylam: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal