Abstract
The ataxia telangiectasia and RAD3-related (ATR) protein kinase is a component of the cellular DNA damage response pathway and promotes cell survival by signalling repair of collapsed replication forks generated by replication stress. We hypothesised that inhibition of ATR potentiates the anti-leukaemic activity of chain terminating nucleoside analogues used in the treatment of acute myeloid leukaemia (AML). We used VE-821 and its derivative VX-970 (Vertex Pharmaceuticals, Abingdon, UK) as potent and specific inhibitors of ATR kinase activity to examine the effects of ATR inhibition in AML cell lines, primary AML cells and AML xenografts.
Co-treatment with 1mM VE-821 did not consistently potentiate the anti-proliferative effects of cytarabine, clofarabine or fludarabine in a panel of AML cell lines. However, there was consistent potentiation of hydroxyurea and gemcitabine in all 7 AML cell lines tested. Treatment with hydroxyurea, which induces replication stress via depletion of dNTPs, resulted in phosphorylation of CHK1, a downstream target of ATR. CHK1 phosphorylation was attenuated when 1mM VE-821 was co-administered with hydroxyurea. Exposure of cells to gemcitabine or hydroxyurea slowed transit through S phase, which was pronounced in combination with VE-821. HL-60 AML cell clones expressing either a constitutively active or inducible shRNA construct targeting ATR had reduced ATR protein expression compared to control cells and were significantly more sensitive to the anti-proliferative effects of gemcitabine and hydroxyurea, but not to cytarabine, clofarabine or fludarabine.
The growth inhibitory effects of hydroxyurea and gemcitabine were also significantly potentiated by VE-821 in primary AML patient samples, which included three adult patients with de novo AML and a paediatric patient with therapy-related AML. In contrast, ATR inhibition did not potentiate the inhibitory effects of hydroxyurea or gemcitabine in primary bone marrow cells from healthy donors ex vivo.
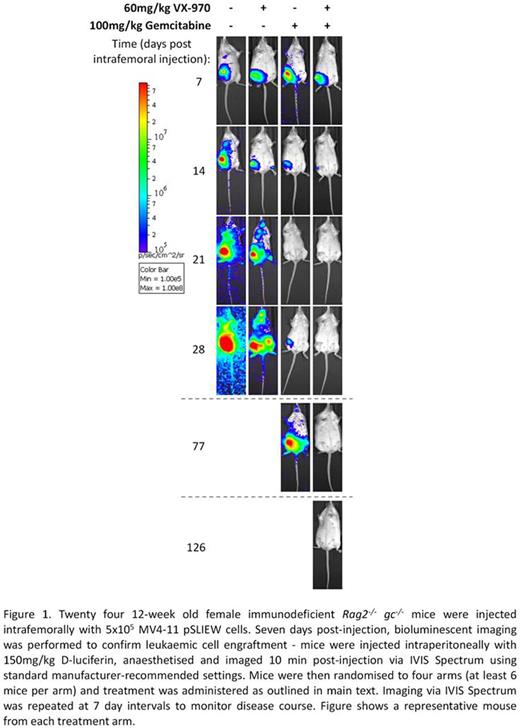
We next sought to determine whether ATR inhibition potentiated hydroxyurea and gemcitabine in an orthotopic mouse model of AML. MV4-11 AML cells engineered to express firefly luciferase (MV4-11 pSLIEW) were intrafemorally transplanted into immunodeficient Rag2-/- gc-/- mice. Bioluminescent imaging via IVIS Spectrum (PerkinElmer, Buckinghamshire, UK) demonstrated localised femoral engraftment first detectable 4-5 days post-injection, with luciferase signal developing in other parts of the body (liver, ovaries) between days 15 and 18 in untreated mice. Treatment was initiated 7 days post-injection when disease was localised to the femur and prior to emergence of disseminated luciferase signal. Single agent hydroxyurea (250mg per kg, IP days 0-4 and 7-11) conferred some early disease control compared to controls as determined by luciferase total body flux measured on day 14, but this was not statistically significant (p=0.18) and did not affect overall survival (mean 35 days for controls and 37 days for hydroxyurea, p=0.47). Monotherapy with VX-970 (60 mg per kg, orally on days 0-4 and 7-11) also conferred early disease control compared to vehicle-treated mice (p=0.18), and resulted in significantly longer overall survival (mean 40 days, p=0.017). Combination treatment with hydroxyurea and VX-970 did not result in more effective early disease control or improved overall survival compared to monotherapy with either agent. Treatment with gemcitabine monotherapy (100 mg per kg, intraperitoneal injection on days 0, 3, 7 and 10) conferred significant early disease control (p=0.002) and significantly improved overall survival compared to controls (mean survival 73 days, p<0.001). Nevertheless, leukemic cells persisted and the cause of death in all mice randomised to this arm was relapsed disseminated disease. Treatment with gemcitabine and VX-970 in combination also conferred very significant early disease control (p=0.002), which eradicated luciferase signal by day 21 in all 6 mice randomised to this arm (Figure 1), and also conferred significantly improved overall survival compared to controls (p<0.001) or mice treated with gemcitabine monotherapy (p=0.001), with four mice remaining disease free at day 126. No significant weight loss or toxicity was observed in any of the treatment arms. Taken together, these data suggest ATR inhibition as a promising therapeutic strategy in AML.
Pollard:Vertex Pharmaceuticals (Europe) Ltd.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal