Abstract
Internal tandem duplications (ITD) are the most commonly observed FLT3 mutations in AML and are associated with decreased survival. In order to achieve meaningful clinical responses with FLT3 inhibitors, sustained inhibition of FLT3 must be achieved [Pratz, Blood 2009]. Quizartinib (Q) is an oral, potent and selective FLT3 inhibitor with an IC50 ≤ 1 nM. In a Phase (Ph) 1 study, the maximum tolerated dose was 200 mg/day (d) in patients (pt) with R/R AML. QTc prolongation was the dose limiting toxicity (Cortes, JCO 2013). In Ph 2 trials, QTc prolongation >500 msec was observed in 15%-17% of pt treated with Q doses of 90 mg/d and 135 mg/d (Cortes ASCO 2013, Martinelli, ASCO 2013). Composite complete response (CRc) rates and overall survival (OS) in FLT3-ITD(+) pt in these studies were similar, respectively. (Levis EHA 2013)] Therefore, we conducted a pre-clinical, pharmacokinetic (PK), and clinical study to find an optimal dosing strategy balancing efficacy with safety for use in future trials.
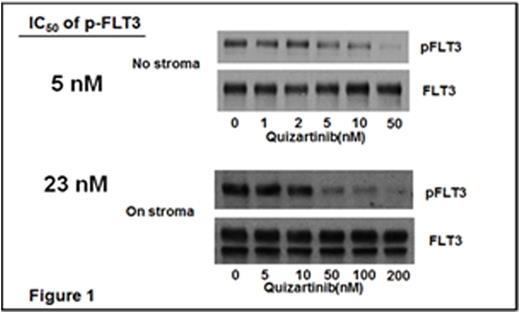
FLT3-inhibitory activity of Q and its major active metabolite with comparable potency, AC886, in human plasma was determined in a FLT3-ITD(+) cell line incubated in human plasma. Results indicated the minimum plasma concentration to fully inhibit FLT3-ITD in peripheral blood (PB) blasts was 200 nM. Less potent FLT3 inhibitors are able to clear blasts from PB when used at MTD, but often have little effect on bone marrow (BM) blasts. Factors contributing to resistance in the BM microenvironment include local metabolism of drug by stromal cell-cytochrome P450 enzymes (Ghiaur, PNAS 2013) and local production of FLT3 ligand (Sato, Blood 2011). To estimate the concentration of Q necessary to inhibit FLT3-ITD in marrow blasts, we conducted concentration-response studies in blasts in suspension culture (as a parallel to PB blasts) vs co-cultured on BM stromal cells. Approximately 5-fold higher levels of Q were needed to achieve the same IC50 in blasts on BM stroma (Figure 1). These results predict that plasma levels between 500 nM-1000 nM would be required to reach complete inhibition of leukemic growth in BM.
The PK of 30 mg and 60 mg doses of Q were evaluated in a Ph 2 study of 76 pt with FLT3-ITD(+) R/R AML. PK dose-proportionality was observed, and steady state (SS) was reached by Day 15. The SS geometric mean trough concentrations for Q+AC886 were 346 nM and 947 nM for 30 mg and 60 mg, respectively. FLT3 inhibition in peripheral blood blasts, as determined both directly and by a plasma inhibitory activity (PIA) assay, was comparable between the 2 doses.
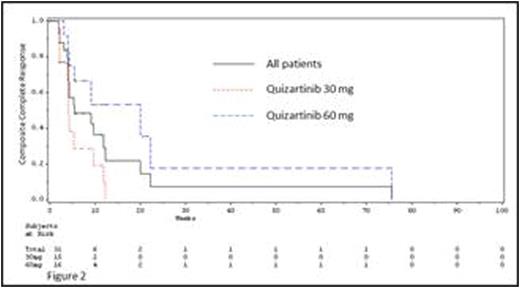
With regards to clinical activity, a trend for longer OS and duration of CRc (DOR) (Figure 2) was seen with the higher dose: median OS 20.9 and 25.4 wk and median DOR 4.1 (95% CI 3.9, 9.7) and 20.0 wk (95% CI, 4.3, 20) for 30 mg/d and 60 mg/d, respectively. A similar trend was observed when these were compared for high and low PK exposures. Higher exposure with 60 mg/d did not result in increased CRc rate (47% both doses). (Cortes, ASH 2013)
In the Ph 2 study, a linear mixed effect model was applied to Fridericia-corrected QT data (QTcF) and time-matched Q and AC886 plasma concentrations to obtain a slope estimate. The model showed that Q plasma concentration, but not AC886, was the significant predictor for QTc prolongation. The model-predicted upper limit of 90% confidence interval of QTcF change from baseline at geometric mean Cmax of 60 mg (869nM) was 22.6 msec, and for 30 mg (332nM) was 8.64 msec. No apparent dose-response relationship was observed for other safety parameters, including myelosuppression or febrile neutropenia. Thus, neither dose was myelosuppressive, but 60 mg/d was associated with somewhat more QTcF prolongation.
This novel laboratory and clinical study has identified 60mg/d of Q as the target dose for clinical efficacy as monotherapy. Although differences in DOR and OS were not statistically significant between 30 mg and 60 mg doses, the sample size was small, and trends for increased benefit were observed at the higher dose. In addition, 60 mg/d provides plasma concentrations (Q+AC886) of 500-1000 nM which are predicted to achieve more complete inhibition of BM blasts. To mitigate the risk of QTc prolongation, a dosing regimen that includes a 30 mg/d lead-in period until SS is reached to allow for QT assessment, followed by escalation to 60 mg/d if tolerated, has been adopted for the Ph 3 study of Q monotherapy in FLT3-ITD-(+) R/R AML (QuANTUM-R, NCT02039726).
Levis:Millennium: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy, Honoraria; Astellas: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Gammon:Daichi Sankyo Pharma Development: Employment. Trone:Daichi Sankyo Pharma Development: Employment. Kang:Daichi Sankyo Pharma Development: Employment. Li:Daichi Sankyo Pharma Development: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal