Abstract
Objective: Determine efficacy and safety of PEMD (pegaspargase, etoposide, methotrexate, dexamethasone) in persons with newly-diagnosed advanced-stage extra-nodal NK/T-cell lymphoma.
Subjects and methods: Twenty-seven consecutive subjects with newly-diagnosed advanced-stage (stage III-IV) extra-nodal NK/T-cell lymphoma were prospectively studied from July, 2010 to August, 2015. All subjects received PEMD (methotrexate, 3.0 g/m2 IV over 6 h on day 1, etoposide, 100 mg/m2 IV on days 2-4, dexamethasone, 40 mg IV on days 1-4 and pegaspargase, 2500 U/m2 IM on day 2). Courses were given every 3 week. Primary co-endpoints were response and survival. Secondary endpoints were proportion of subjects completing planned therapy (4 to 6 cycles of PEMD regimen) and frequencies of adverse events.
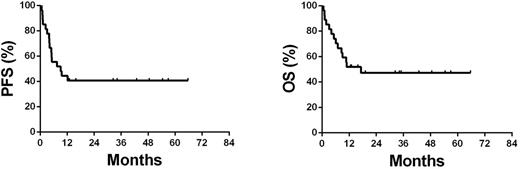
Results: Median age was 46 y (range, 17-73 y). There were 21 males (78%). Nine subjects (33%) had non-nasal NK/T-cell lymphoma including 5 of the skin involvement, 1 of the testis involvement, 1 of the muscle involvement and 2 of the gastrointestinal tract involvement. Thirteen subjects (48%) had elevated serum LDH levels. Eleven subjects (41%) had higher level of EBV-DNA in blood (>5000 copies/mL). Twenty-one subjects (78%) had B-symptoms and 13 (48%) had an IPI score of 3-5. Subjects received a median of 4 courses of PEMD (range, 1-6). Median follow-up is 48 mo (range, 13-74 mo). Three patients had early death (within 3 mo after the diagnosis). Overall response rate (ORR) was 74% (95%CI 54%-89%) in the 27 subjects with advanced-stage disease including complete response (CR)/unconfirmed CR (CRu) in 12 (44% [95%CI 26%-65%]) and a partial response (PR) in 8 (30% [95%CI 14%-50%]). Four-year progression-free survival (PFS) was 44% (95%CI 25%-63%) and overall survival (OS) 51% (95%CI 32%-70%) (Figure 1). PFS and OS were not correlated between nasal and non-nasal types of ENKTL. There was no treatment-related death or serious allergic reactions. The most common grade-3/-4 hematologic complication was neutropenia (37% [95%CI 19%-58%]). The most common non-hematologic complications were infection (16% [95%CI 4%-34%]) and hypo-fibrinogenemia (12% [95%CI 2%-29%]).
Conclusion: PEMD is effective and safe in persons with newly-diagnosed advanced-stage extra-nodal NK/T-cell lymphoma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal