Abstract
Introduction
The Bruton's tyrosine kinase (BTK) inhibitor ibrutinib has transformed the management of patients with CLL, yet it does not induce substantial apoptosis in vitro. The mechanisms underlying the ability of BTK inhibitors to kill CLL cells are incompletely understood. Our group previously developed BH3 profiling, a functional assay that determines the proximity of a cell to the threshold of apoptosis, a property called "mitochondrial priming". A new variant of this technique, dynamic BH3 profiling (DBP), measures early drug-induced changes in mitochondrial apoptotic signaling before cell death occurs. Using DBP, we previously found that ibrutinib increases mitochondrial BCL-2 dependence in CLL cells, thereby sensitizing cells to the BCL-2 inhibitor venetoclax (formerly ABT199/GDC-0199). However, it remains unclear whether these are on-target effects of ibrutinib on BTK or off-target effects of ibrutinib on its other targets such as ITK or TEC kinase. Here, we examined acalabrutinib, a 2ndgeneration, highly specific BTK inhibitor currently in clinical trials to determine whether selective BTK inhibition increases BCL-2 dependence in CLL cells.
Methods
Mononuclear cells were isolated from CLL patients and treated ex vivo with acalabrutinib (provided by Acerta) alone or in combination with venetoclax (APEX Bio Technology) in the presence of NKTert stromal cells. BH3 profiling and dynamic BH3 profiling were then performed as previously described by measuring the release of cytochrome c on a BD FACS Fortessa (Montero J. et al. Cell 2015). Cell viability assays and western blot analyses were performed according to standard protocols. The student's t-test or one-way ANOVA was used to compare drug treatments with p<=0.05 considered significant.
Results
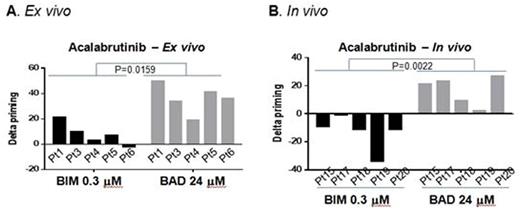
To validate our prior finding that ibrutinib increased mitochondrial BCL-2 dependence, we first repeated our prior ex vivo ibrutinib experiments in 3 additional patients and found similar results. Next, we performed DBP on 5 peripheral blood samples from previously untreated CLL patients using ex vivo treatment with acalabrutinib, venetoclax or the combination. Consistent with our findings with ibrutinib treatment, acalabrutinb increased BCL-2 dependence, with all patient samples showing >20% increase in priming ("delta priming") in response to BAD BH3 peptide, with no increase in overall priming (<20% in all patients) measured by BIM BH3 peptide (Figure 1A). Moreover, it did not alter BCL-xL or MCL1 dependence measured by their specific peptides HRK or MS1, respectively. In contrast, venetoclax increased the overall level of mitochondrial priming with minimal effects on BCL-2 dependence. When combining these two drugs, both overall priming and BCL-2 dependence increased. The increase in BCL-2 dependence by ibrutinib resulted in enhanced sensitivity to venetoclax (p=0.0005).
To assess whether these ex vivo effects could be replicated in vivo, we next performed DBP using CLL patient samples from 5 patients on the acalabrutinib first-in-human phase I clinical trial (NCT02029443). Delta priming was calculated by comparing pre-treatment samples to samples obtained after 4 weeks of acalabrutinib monotherapy. Consistent with our ex vivo data, acalabrutinib therapy selectively increased mitochondrial BCL-2 dependence in vivo (Figure 1B) without altering BCL-XL or MCL-1 dependence, and overall priming decreased slightly. To provide additional insight into the molecular mechanism underlying the mitochondrial priming changes, we did western blot analyses for BCL-2 family proteins, and found that expression of the pro-apoptotic BIM was increased after acalabrutinib therapy.
Conclusions
Selective BTK inhibition with acalabrutinib enhances mitochondrial BCL-2 dependence and increases CLL cell sensitivity to the BCL-2 inhibitor venetoclax. Induction of expression of the pro-apoptotic protein BIM provides an initial clue as to a mechanism underlying these effects. These data strongly suggest that the increase in BCL-2 dependence we previously found with ibrutinib is a BTK-specific effect. Our findings provide a strong pre-clinical rationale supporting clinical trials combining BTK and BCL-2 inhibition. More broadly, we also demonstrate that DBP can be utilized on in vivo treated patient samples to rationally construct combination regimens of targeted agents to treat cancer.
Brown:Pfizer: Consultancy; Sun BioPharma: Consultancy; Celgene: Consultancy; Roche/Genentech: Consultancy; Gilead Sciences: Consultancy; Janssen: Consultancy; Infinity: Consultancy; Abbvie: Consultancy. Letai:AbbVie: Consultancy, Research Funding; Astra-Zeneca: Consultancy, Research Funding; Tetralogic: Consultancy, Research Funding. Davids:Gilead: Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; TG Therapeutics: Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Infinity: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal