Abstract
Background: A challenge for novel therapeutic strategies will be the fine tuning of intracellular reactive oxygen species (ROS) signaling in order to effectively deprive cells from ROS-induced tumor promoting events and subsequent tipping the balance to ROS-induced apoptotic signaling. ROS plays a critical role in regulation of the pro-survival receptor tyrosine kinase (RTK) signaling pathways in human cancers. Studies have identified mitochondrial metabolism as the key source for abundant ROS in chronic lymphocytic leukemia (CLL). Unlike in other malignant cells, increased oxidative phosphorylation but not increased aerobic glycolysis has been found in CLL B-cells. While we have detected constitutively phosphorylated/active RTKs like Axl in CLL B-cells, the mechanism of activation remains largely undefined. Here we report that (i) elevated ROS activates the Axl signaling pathway in CLL B-cells and, (ii) mechanism of increased ROS accumulation in the leukemic B-cells.
Methods: CLL B- and normal B-cells were purified from peripheral blood of previously untreated CLL patients and normal healthy individuals, respectively using a RossetteSep B-Cell enrichment kit. CLL B-cells were exposed to H2O2 for 5 min and cell lysates were analyzed for activation of RTKs including Axl by immunoprecipitation/Western blots. We also examined expression status of SIRT3, acetylated-superoxide dismutase (SOD)2 and catalase in CLL B-cells by Western blots. Catalase mRNA levels in CLL B-/normal B-cells were determined by qRT-PCR. In some experiments, genomic DNA was isolated from CLL B-/normal B-cells for catalase promoter methylation studies. Finally, accumulation of O2‾ and H2O2 in CLL B- and normal B-cells were measured by flow cytometry after treating the cells withdihydroethidium (DHE) and dichlorohydrofluorescein diacetate (DCFDA), respectively.
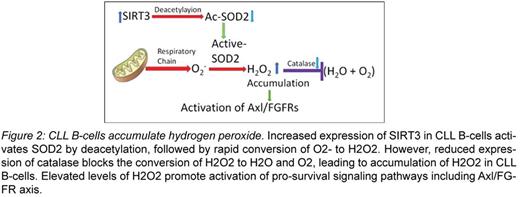
Results: We found that enforced induction of ROS significantly increases tyrosine phosphorylation levels on multiple cellular proteins in CLL B-cells (Fig. 1). Further analysis finds that increased ROS activates Axl and its downstream targets AKT/Erk1/2 MAPK and the fibroblast growth factor receptor (FGFR) which we recently defined as a downstream target of Axl; while ROS accumulation did not show any significant effect on other RTKs, cMET or IGFR1, in CLL B-cells. Interestingly, the histone deacetylase SIRT3 which activates mitochondrial SOD2 via deacetylation, we found, was overexpressed in CLL B-cells as compared to normal B-cells indicating more efficient conversion of O2‾ into H2O2 in the leukemic B-cells (Fig. 2). However expression of catalase, which converts H2O2 into O2 and H2O, was reduced significantly in CLL B-cells as compared to normal B-cells both at mRNA and protein levels. To delineate the mechanism of reduced expression of catalase in CLL B-cells we studied the human catalase promoter region for potential methylation sites using the method of HpaII restriction enzyme digestion where HpaII is unable to cut DNA (CCGG site) when the internal cytosine is methylated. Initial findings from this approach suggest that while the catalase gene promoter in CLL B-cells may contain methylated cytosines, the catalase gene in normal B-cells may not confer methylation in the promoter region. Next, genomic DNA from CLL B-cells (n=10) and normal B-cells (n=6) was subjected to bisulfite treatment, followed by PCR amplification and sequencing. This approach revealed no significant promoter methylation of the catalase gene in normal B-cells, while moderate to heavily methylated promoter of the catalase gene was detected in CLL B-cells from majority of CLL patients (9 of 10). Further studies are in progress to examine catalase promoter methylation status in a larger cohort of CLL patients and its association with H2O2 accumulation, RTK phosphorylation and risk-factors. Indeed, flow cytometric analysis finds lower levels of O2‾ but accumulation of H2O2 in CLL B-cells as compared to normal B-cells. Taken together, these findings suggest that although SOD2 remains highly active, increased accumulation of H2O2 may occur in CLL B-cells due to epigenetic silencing of the catalase gene.
Conclusion: These observations may explain, at least in part, the expression of constitutively active Axl signaling pathway in CLL B-cells that plays a role in CLL B cell survival and may account for the prolonged survival and/or apoptotic resistance of the leukemic B-cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal