Abstract
Background
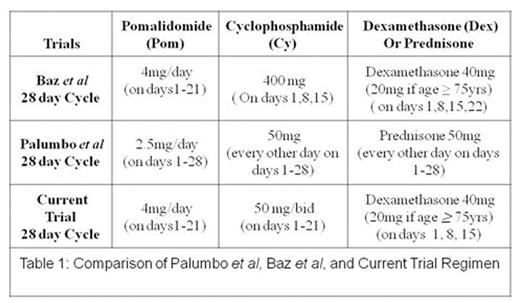
A treatment option for patients with relapsed/refractory multiple myeloma (RRMM) is pomalidomide(pom) and dexamethasone (dex), with an overall response rate (ORR) of 33% and median progression free survival (PFS) of 4.2 months. Adding the alkylatingagent cyclophosphamide(Cy) to pom and steroids improves ORR and PFS. Baz et al (Blood, 26 May 2016) combined daily pom with weekly dosing ofCy anddex (PCD), with an ORR of 64.7% and a median PFS of 9.5 months, although grade 3/4 neutropenia increased from 31% to 52%.
In our experience, compared to weekly Cy, low dose daily oral Cy is better tolerated with less myelosuppression. Palumbo et al (Blood, 17 Oct 2013) in fact combined pom with alternate day dosing ofCy and prednisone, with an ORR of 51% and a median PFS of 10.4 months and a grade 3/4 neutropenia rate of 42%. However, importantly, granulocyte stimulating factor (G-CSF) and platelet transfusion support wereprohibited, resulting in a lower maximum tolerated dose of pom of 2.5 mg (vs 4 mg in the Baz) and therefore, the rates of neutropenia cannot be compared between the two studies.
In the present study, we explored PCD at the doses/schedule shown in table 1 with hematologic support even in patients with baselinecytopenias. This type of metronomic therapy has demonstrated efficacy in refractory B cell malignancies, possibly because the anti-angiogenic effects of metronomic therapy may be synergetic with conventional anti-neoplastic agents.
Methods
This was an open label, single arm, and single center phase 2study. The primary objective was to evaluate the best ORR. Secondary objectives were to evaluate safety, clinical benefit response (CBR), PFS, and overall survival (OS).
Inclusion criteria included lenalidomide refractory, pom naïve RRMM patients with at least 2 prior lines of therapy. Patients were required to have measurable disease, adequate performance status, Cr <3 mg/dL, normal hepatic function, and ANC > 1000/uL and platelets > 50,000/uL if bone marrow plasma cells were < 50%, otherwise >30,000/uL. G-CSF and platelet support were permitted during screening and study treatment if needed. Each drug was administered at the doses and schedule shown in Table 1.
Results
Overall, 28 evaluable patients with progressive disease (PD) at screening have been enrolled. The median age is 66 (57% > 65 yr) with a median of 3 lines of prior therapy over 5 years since diagnosis. 3 (11%) had ANC<1.5 and 2 (7%) hadplts<50,000/µL at study entry.High-risk molecular findings were present in 13 patients (46%), including 3 with del p53 and 6 with gain of 1q21 by FISH (2 with concurrentt(4;14) and 2 with concurrent del p53).
With 8 patients still on study therapy, responses include 3 complete responses (CR), 7 very good partial responses (VGPR), 9 partial responses (PR), 3 minor responses (MR), 5 stable disease (SD), and 1 PD, for an ORR of 67%, CBR (i.e. MR or better) of 78% and a median PFS of approximately 14.5 months. The median OS has not been reached.
The most common grade 3/4 toxicity (regardless of drug attribution) was neutropenia with 20 (71%) of subjects experienced grade 3/4 neutropenia. Importantly, there was only 1 episode of febrile neutropenia during study therapy. Grade 3/4 thrombocytopenia was seen in 25% of subjects, and 3/4 anemia seen in 18%.
The most common grade 3/4 non-hematologic toxicity was pulmonary disease with Grade 3 lung infections occurring in 21% of subjects (3 viral, 2 bacterial, 1 unknown) and 1 additional grade 3 URI. Of note, all of these admissions occurred at local hospitals and none of these occurred in the setting of neutropenia. One additional pt hadpneumonitisattributed to pom requiring study discontinuation. Grade 3rashwas also observed in 14% of subjects leading to pom dose reductions.
Correlative data from peripheral blood and bone marrow aspirates taken at baseline, Cycle 3 Day 15, and at disease progression from all patients will be updated at the time of conference. These include PCD-associated changesin gene expression, clonal evolution and immune microenvironment during therapy and on progression.
Conclusions
With toxicities similar to those in other studies, the ORR of 67% and PFS of 14 months in our study of PCD compares very favorably to pomdexas well as other triplet regimens containingCy.
Chari:Takeda: Consultancy, Research Funding; Array Biopharma: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Pharmacyclics: Research Funding; Amgen Inc.: Honoraria, Research Funding. Cho:Genentech Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Research Funding; Agenus, Inc.: Research Funding; Ludwig Institute for Cancer Research: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding. Catamero:Celgene: Honoraria, Speakers Bureau. Verina:Celgene: Speakers Bureau. Jagannath:Bristol Myer Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal