Abstract
Background:
Targeted monoclonal antibodies (mAbs) such as the anti-CD20 rituximab, are proven therapies in lymphoma, yet these diseases remain incurable because of primary or acquired resistance. Using a eukaryotic expression system to produce antigen closely representing endogenous protein, we developed a new therapeutic antibody against an alternative lymphoma target. B cell activating factor receptor (BAFF-R/TNFRSF13C) is a tumor-necrosis factor receptor superfamily member specifically involved in B lymphocyte development and mature B cell survival. Although earlier attempts to target the BAFF/BAFF-R axis therapeutically for B cell tumors yielded limited success, BAFF-R remains an attractive target for B cell lymphoma therapeutic antibody development, particularly for rituximab-resistant tumors.
Methods and Results:
We generated 2 mAbs to human BAFF-R expressed as a natively folded, eukaryotically glycosylated cell-surface immunogen on engineered mouse fibroblast (L) cells. Both mAbs specifically bound BAFF-R-expressing L cells, but not the parental counterparts. Each of the complementarity determining regions (CDRs) of the 2 mAbs are unique, suggesting different binding epitopes. Both mAbs bound with high affinity to the human B cell lymphoma cell lines JeKo-1 (mantle cell lymphoma; MCL), SU-DHL6 (diffuse large B cell lymphoma; DLBCL), Raji (Burkitt's lymphoma) and RL (follicular lymphoma). Because our goal is to develop antibodies for clinical use, we substituted in human IgG1 Fc to generate the chimeric mAbs C55 and C90. The chimeric mAbs retained the binding specificity and affinity of the mouse antibodies to their target cells. By immunohistochemistry C55 and C90 staining was specific to the B cell-containing organs tonsil and spleen. No detectable staining was observed in heart, lung, brain, liver, and kidney. Using primary human natural killer (NK) cells as effectors, we demonstrated the chimeric antibodies induced potent antibody-dependent cellular cytotoxicity (ADCC) against BAFF-R-expressing L cells and the JeKo-1, SU-DHL6, Raji and RL human lymphoma cell lines. C55 and C90 were also able to elicit ADCC in primary human lymphomas; they efficiently killed tumor cells from patients with MCL, DLBCL, follicular lymphoma and chronic lymphocytic leukemia (CLL) (n=8). Notably, 5 of these primary lymphomas were from patients who had relapsed after rituximab treatment (2 MCL, 3 CLL).
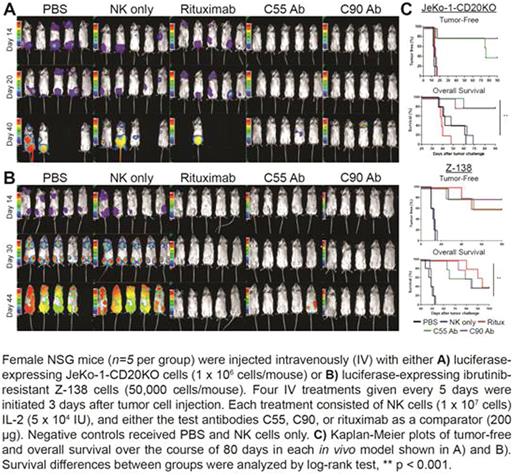
We next determined the activity of C55 and C90 in models of drug-resistant lymphoma. Both ibrutinib and rituximab are effective anti-lymphoma agents, however, primary or acquired resistance to these drugs is common. We derived a rituximab-resistant JeKo-1 variant (JeKo-1 CD20KO) using the CRISPR/HDR system to knock-out CD20 gene expression, and also used the naturally ibrutinib-resistant (Z-138) lymphoma cell line. We confirmed that the C55 and C90 anti-BAFF-R antibodies induced ADCC in both drug-resistant cell lines in vitro. Using xenogenic tumor models in NOD scid gamma (NSG) mice we observed remarkable in vivo anti-tumor effects of both the C55 and C90 chimeric antibodies. We found our antibodies significantly inhibited growth of implanted Z-138 and JeKo-1 CD20KO lymphomas (P<0.001; Figure).
Conclusion:
In contrast to previously reported BAFF-R antibodies, our in vitro and in vivo results strongly support the translational development of our novel BAFF-R-specific monoclonal antibodies, especially as an alternative immunotherapy against ribuximab- or ibrunitib-resisitant B cell maglinancies. Other preliminary data also suggest BAFF-R may be an effective target of CAR T cells.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal