Abstract
Background:Poor graft function (PGF) is an uncommon but significant complication of hematopoietic cell transplantation (HCT). There is growing interest in treatment with positively selected CD34+grafts as "stem cell boosts" (SCB). No data are, to our knowledge, available concerning the use of SCBs in the HLA-haploidentical setting.
Methods:We retrospectively identified all patients undergoing SCB after peripheral blood haplo-HCT with post-transplant cyclophosphamide at our institution between June 2009 and January 2016. CD34+ cell selection was carried out on the CliniMACS instrument (Miltenyi Biotec, Auburn, CA). PGF was defined as ≥95% donor chimerism, and at least two of the following three cytopenias for at least 2 consecutive weeks without alternative explanation: ANC < 1500 cells/mm3, platelets < 30,000/mm3 and Hgb < 8.5 g/dL. PGF was classified as primary if it occurred immediately following day +14. Otherwise, it was considered secondary. Patients with 5-95% donor chimerism and ANC > 500 cells/mm3 who otherwise met the criteria for PGF were classified as PGF with mixed chimerism. Hematologic improvement (HI) was defined as having no cytopenias. Hematological response (HR) was defined as ANC > 2500 cells/mm3, platelets >100,000/mm3, and Hgb >10 g/dL. Both HI and HR required transfusion independence for the previous 30 days. Acute and chronic graft-versus-host disease (GVHD) were graded based on established criteria. Overall survival (OS) was defined as time from SCB until death or last clinical contact.
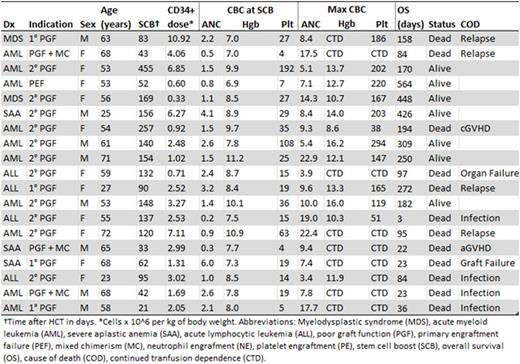
Results:Nineteen of 141 patients (13%) undergoing haplo-HCT received SCB. Clinical characteristics are summarized in Table 1. SCB was given at a median of 120 days (range: 21 - 455) after haplo-HCT. Median time from SCB to death or last clinical contact was 158 days (range: 3 - 564). Nine donors underwent remobilization; 10 patients used cryopreserved product. The median SCB contained 2.52 x 106 CD34+ cells/kg (range: 0.33 - 10.92). SCBs from remobilized donors contained significantly more CD34+ cells/kg (median: 4.06) than those from original mobilization (median: 1.50) (p<0.01). Median TNC dose was 0.03 x 108 cells/kg (range: 0.01 - 0.73). All CD3+ cell doses were < 0.01 x 107cells/kg.
At time of SCB, 63% (12/19) patients were cytopenic in at least two cell lines and 26% (5/19) were cytopenic in all three cell lines. Six patients had one cytopenia and one had no cytopenias, but was chronically transfusion dependent. The majority (74%) of patients had hypocellular bone marrows per pathology report. Patients with secondary PGF were less likely to have multiple cytopenias than the remaining cohort (36% versus 100%, p=0.01). No patients received conditioning prior to SCB. Eighteen patients were receiving immunosuppression, including steroids (79%), calcineurin inhibitors (63%) and mycophenolate mofetil (32%).
At day +90 after SCB, 66% of surviving patients were in HI or HR.. ANC was significantly higher at day +90 (p=0.02). Hgb and platelets were significantly higher at all time points (p<0.05). No association between HR or HI and SCB CD34+ cell dose was observed. Median OS was 158 days. Median survival in patients with multiple cytopenias at SCB was 84 days, while it was not reached for those with ≤1 cytopenias. At day +90, 80% of patients with secondary PGF were alive, compared to 50% with primary PGF and 0% with PGF and mixed chimerism. In patients surviving to day +90, survival was significantly better at last follow-up among patients achieving HR or HI (88% vs. 0%, p=0.01). One patient developed new stage 2 aGVHD of the gut following SCB. Three patients developed mild-to-moderate cGVHD at 88, 128 and 130 days after SCB.
Conclusion:Our results suggest that SCB after haplo-HCT is safe and associated with acceptable rates of acute and chronic GVHD. It may also be effective in reducing transfusions and inducing HI and HR. The treatment of these patients continues to be a challenge. Further studies are needed to delineate the factors influencing outcomes in this population, including timing of therapy and optimal CD34+ cell dose.
DiPersio:Incyte Corporation: Research Funding. Vij:Celgene: Consultancy; Karyopharm: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Takeda: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria. Fehniger:Celgene: Research Funding; Affimed: Consultancy; Fortress Biotech: Consultancy. Schroeder:Incyte Corporation: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal