Abstract
Background
Surface decoration of Hb with PEG chains has been advanced as an approach to attenuate the vasoconstrictive activity of acellular Hb in circulation. PEGylated Hbs with higher oxygen affinities relative to unmodified Hb has an advantage when designing blood substitutes in achieving targeted transport of O2 from lungs to tissues. Extension Arm Facilitated (EAF) PEGylation of Hb with six copies of PEG 5K (EAF P5K6 Hb) was advanced as an optimum level of PEGylation that does not induce weakening of interdimeric interactions in the quaternary structure of Hb. EAF P5K6 Hb at 4 gm % is an O2 carrying colloidal plasma expander. However, a prototype of EAF P5K6 Hb, developed by Sangart as MP4, did not increase microvascular oxygen delivery or improve tissue oxygenation in models of extreme hemodilution,whereas the EAF P5K6 Hb and P5K2 did better than the non-oxygen carrying semisynthetic colloidal plasma expander and EAF P5K6 albumin.Also, P5K2 Hb does a better tissue oxygenation even though its oxygen affinity is comparable to that of EAF P5K6 Hb1.
As PEG chains increases from 2 to 10 copies, there is a lesser effect in the oxygen affinity of PEG Hbs in the presence of allosteric effectors like IHP and L-35. This suggests that higher degree of PEGylation preferentially stabilize the oxy conformation of PEG Hb, decreasing the efficacy of O2 release in the circulation. We attributed this decreased efficiency to the larger PEG shell which might lower the diffusion of O2out to the plasma. We reasoned that we could reduce the size of the PEG shell using six copies of PEG 3K and that we could use this new PEG Hb (EAF P3K6 Hb) at 6 gm% to get nearly the same viscosity as 4 gm % EAF P5K6 Hb. We compared tissue oxygenation by EAF P3K6 Hb with EAF P5K6 Hb and P5K2 Hb in an experimentally induced anemia model and tested its therapeutic efficacy in attenuating the experimentally induced acute crisis in a mild murine model of SCD, NY1DD.
Methods
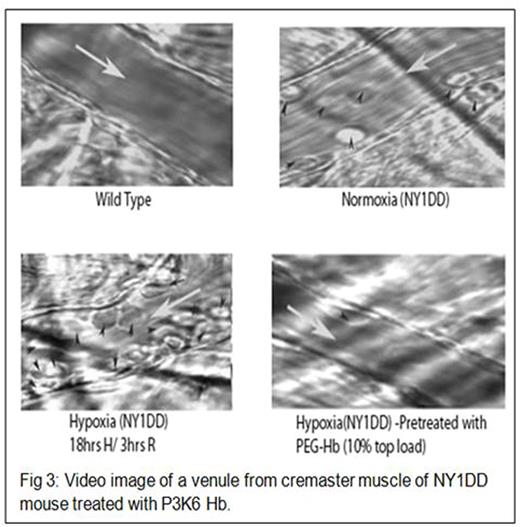
EAF P3K6 Hb was prepared with PEG3K and characterized as EAF P5K6 Hb2. Molecular models and the shapes of PEG Hbs have been developed using InsightII program (Accelrys). Tissue oxygenation studies were carried out in the extreme hemodilution hamster model as described previously.1 Acute vaso-occlusion was studied using intravital microscopy in the venules of cremaster muscles of NY1DD SCD mice subjected to hypoxia-reoxygenation (18 hrs of hypoxia with 8% O2 and then normoxia).3The efficacy was measured after treatment with 10% top load of 6 gm % solution of EAF P3K6 Hb, given at the transitioning stage from hypoxia to normoxia
Results
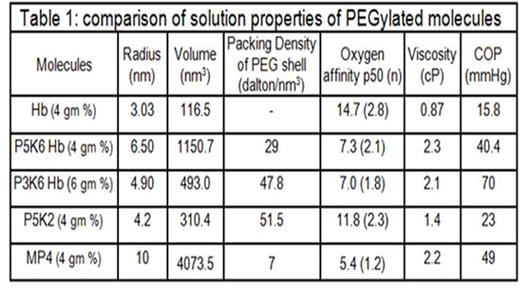
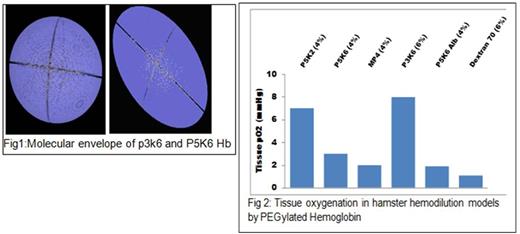
PEG 3K chains reduced the molecular radius and volume while increased the packing density of PEG shell of EAF P3K6 Hb as compared to EAF P5K6 Hb (Table 1). The packing of the PEG chains induced a globular shape to the EAF P3K6 Hb vs ellipsoidal shape to EAF P5K6 Hb (Fig 1). Oxygen affinity of EAF P3K6 was comparable to that of EAF P5K6 Hb. In the extreme hemodilution hamster model of anemia, the efficacy of EAF P3K6 Hb to increase tissue oxygenation was comparable to that of P5K2 Hb (Fig 2). From the intravital micrographs of cremaster muscles (Fig 3), P3K6 Hb markedly attenuated the induced vaso-occlusive crisis in NY1DD mice.
Discussion and Conclusions
We have previously shown that EAF P3K6 Hb is effective in normalizing the chronic state of vaso-occlusion in BERK sickle mice. We demonstrate here that EAF P3K6 Hb protects NY1DD sickle mice from developing acute vaso-occlusion. These studies suggest that a smaller compact PEG shell around Hb increases the ability of the PEG Hb to oxygenate the tissues. NY1DD mice are not very anemic (Hb > 10 gm/dl); a 10% top load of 6 gm% high oxygen affinity Hb represents no significant increase in Hb level (≈0.06 gm %). We suggest that the ability of such a miniscule amount of PEG Hb in the plasma to protect the animal from hypoxia is due to its enhanced ability to deliver O2 under anemic and hypoxic conditions. We suggest that EAF P3K6 Hb facilitates improved extraction of O2 from RBC to plasma and to the subsequent diffusion to tissues, i.e. small amount of PEG Hb in plasma acts as a catalyst for O2 transfer while the oxyHb in RBC serves as the local reservoir for O2. This improved O2 extractionmay be effective in anemia in general, and have a unique application in sickle cell disease.
References
1.Cabrales, P et al (2004) Am J Physiol Heart Circ Physiol, 287:H1609-H1617.
2. Acharya, S. A. et al (2007). Biochem J,
3.Kaul D.K. et al (2000) J Clin Invest, 106(5):715.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal