Background. Anticoagulation therapy is the cornerstone of acute treatment of venous thromboembolism (VTE) and for prevention of recurrent VTE. The need for anticoagulation is increasing in children, largely in part due to increasing VTE rates. Conventional anticoagulants, including heparin, low-molecular weight heparins (LMWH), Fondaparinux, and vitamin K antagonists (VKA) are widely used in children but have limitations. Standard of care management with these agents is plagued with the trade-off between daily or twice daily injections or frequent monitoring of therapeutic effect. The advent of direct oral anticoagulants (DOACs) have catalyzed significant changes in the therapeutic landscape of VTE management. DOACs have been evaluated for safety and efficacy in large, randomized controlled trials in the treatment and prevention of VTE in adults, with results that are comparable to conventional therapy. None of the current DOACs have FDA-approved indications and dosing in children yet. Off-label use of these agents is largely based on adult data and doses, and is increasing at many Children's Hospitals across US. Rivaroxaban, a DOAC, is a factor Xa inhibitor with predictable pharmacokinetic and pharmacodynamics properties.
Methods. We describe a case series of 8 unique pediatric cases, treated with Rivaroxaban, for a variety of non-routine indications, due either to adverse effects, intolerability of LMWH or VKA or the need for ongoing, long term anticoagulation. Rivaroxaban was started after informed consent and assent from parents or patients respectively, and was initiated at a fixed dose but titrated to a final dose after monitoring of trough and peak Rivaroxaban levels (Aniara, West Chester,OH, USA).
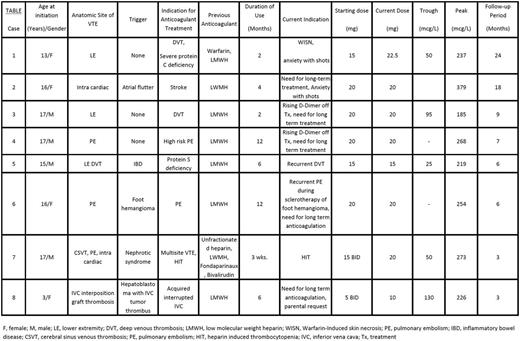
Results. The mean age of patients in this case series is 14 years (median: 16, range 3-17) (see Table). The most common indication to use Rivaroxaban was the need for long term anticoagulation after having completed therapeutic anticoagulation, except in two patients, one of whom developed warfarin skin necrosis due to protein C deficiency and another with heparin induced thrombocytopenia. Only two patients needed dose adjustments to achieve target trough and peak drug levels. The mean duration of follow-up is 9 months (median= 5.5; range 3-24) (see Table) at this time. None of the patients developed recurrent VTE while on Rivaroxaban. A soft tissue traumatic bleed occurred in one patient which was treated with holding off the drug for 48 hours. No other bleeding complications were observed.
Conclusions. Clinical application of DOACs in a real world clinical setting, including strong thrombophilia and malignancy, results in treatment profile of high efficacy and safety in children; however, larger studies are needed to validate these findings.
Sarode:CSL Behring: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal