Abstract
Background: Venous thromboembolism (VTE), bleeding, and thrombocytopenia are common occurrences during treatment of hematological malignancies. When VTE is complicated by severe thrombocytopenia, the safety and efficacy of anticoagulation is unclear.
Methods:Single center retrospective chart review of subjects between 4/2004-12/2015 with active hematological malignancy and either 1) acute or chronic VTE on anticoagulation before platelet count dropped below 50k/µL or 2) acute VTE occurring while platelets were <50k/µL. Primary outcomes: time to recurrent VTE or clinically significant bleeding within 100 days from onset of severe thrombocytopenia. Exclusion criteria: age <18 years, hospice care, heparin-induced thrombocytopenia, thrombotic thrombocytopenic purpura, or contraindication to anticoagulation (excluding thrombocytopenia). Charts were reviewed to determine anticoagulation management strategy during episodes of severe thrombocytopenia and to confirm primary outcomes.Kaplan-Meier curves were created from time to event data for recurrent VTE and clinically significant bleeding by anticoagulation strategy and was censored for death, lost to follow up, and the opposite outcome. Incidence rates (IR) and incidence rate ratios (IRR) were calculated using person time with platelets<50k/µLfor recurrent VTE, clinically significant bleeding, and combined incidence of bleeding or recurrent VTE (composite outcome).
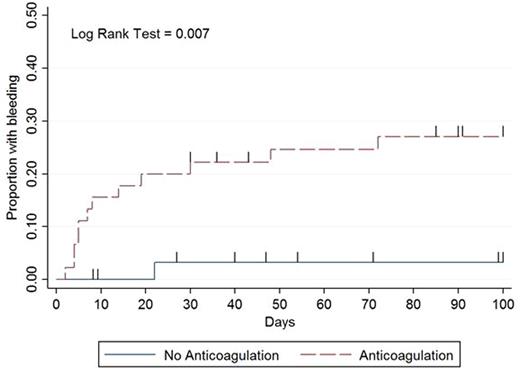
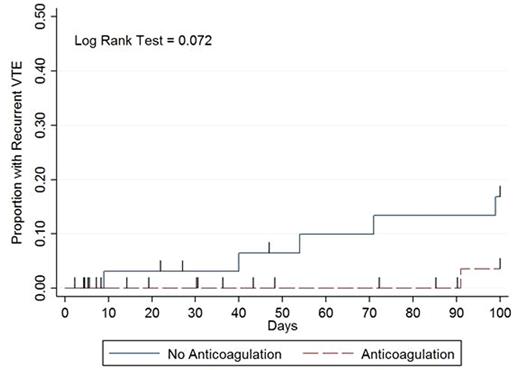
Results: Over 11.5 years, 78 patients met inclusion criteria. Mean age was 53 years (range 19-81) and median duration of severe thrombocytopenia was 14 days (IQR 6-35). The initial treatment strategies when platelets dropped below 50k/µL were (i) therapeutic dose of anticoagulation with supportive platelet transfusions to maintain a count > 40-50,000/µL (n=43, 55%), (ii) no anticoagulation (n=33, 42%), and (iii) reduced or prophylactic dose anticoagulation (n=2, 3%). Clinically significant bleeding occurred in 13 patients with a median number of days to bleed of 8 (interquartile range 5-22) with the majority (n = 11, 85%) occurring on or before day 30 (Figure). Two patients experienced a life threatening bleed. Recurrent VTE occurred in 6 patients with a median number of days to recurrence of 62 (IQR 40-91) with the majority occurring after day 40 (n = 5, 83%: Figure). The median platelet count most proximate to event: bleeding, 38k/µL (range 5k-212k); recurrent VTE, 225k/µL (range 21k-407k). No recurrent VTE or bleeding resulted in death. Clinically significant bleeding within 100 days after the onset of severe thrombocytopenia occurred in 12/45 (27%) patients receiving anticoagulation vs. 1/33 (3%) patients when anticoagulation was held (IRR 17.7, 95% CI 2.62-756.98). Recurrent VTE occurred in 1/45 (2%) patients in the anticoagulation group versus 5/33 (15%) patients when anticoagulation was held (IRR 0.3, 95% CI 0.01-2.64). The composite outcome occurred in 13/45 (29%) patients in the anticoagulation group versus 6/33 (18%) patients when anticoagulation was held (IRR 3.2, 95% CI 1.13-10.26).
Conclusions:The significantly higher rate of major bleeding and composite outcome in patients getting therapeutic dose anticoagulation with supportive platelet transfusions during episodes of severe thrombocytopenia raises concerns over this treatment as the primary management strategy for VTE in patients with hematological malignancies and severe thrombocytopenia. Initially, bleeding risk is high on anticoagulation yet the risk of recurrent VTE is low. However, with time and platelet recovery these risks reverse. The fluctuating risk of bleeding and recurrent VTE over time in patients with hematological malignancies and severe thrombocytopenia illustrates that anticoagulation strategies should vary in aggressiveness depending on degree and duration of thrombocytopenia.Safety and efficacy trials incorporating dynamic risk-adapted anticoagulation strategies are urgently needed to determine the best treatment strategy for VTE occurring in patients with hematologic malignancies and severe thrombocytopenia.
Acknowledgments: Supported by NIH CTSA at the University of North Carolina (UL1TR001111) and UNC Hematology T32 (5T32HL007149-39).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal