Abstract
Introduction: Phosphatidylserine (PS) is a phospholipid normally residing in the inner leaflet of the plasma membrane that becomes exposed on vascular endothelial cells and tumor cells in the tumor microenvironment, particularly in response to chemotherapy and irradiation. Binding of antibodies targeting PS on the tumor endothelial cells and tumors induces the recruitment of immune cells and engages the immune system to destroy tumor and associated vasculature and by blocking the immunosuppressive action of PS. Recent studies have demonstrated that PS-targeting antibodies enhance the anti-tumor activity of immune checkpoint antibody blockade to CTLA-4 and PD-1 in mouse breast and melanoma tumor models (Freimark et al. Cancer Immunol. Res. 2016; Gray et al. Breast Cancer Res 2016). Ibrutinib is an approved anticancer drug targeting B-cell malignancies that is a selective, covalent inhibitor of the enzyme Bruton's tyrosine kinase(BTK) in B-cell tumors. Data from recent mouse tumor studies demonstrate that ibrutinib in combination with anti-PD-1 antibody blockade inhibits growth of solid tumors (lacking BTK expression) suggesting that ibrutinib may inhibit kinases of the immune system such as interleukin-2 inducible T-cell kinase (ITK), to enhance specific anti-tumor responses (Sagiv-Barfli et al. PNAS 20 2015).
Methods: The present study was conducted to evaluate the anti-tumor effects of combination therapy including PS-targeting antibody mouse chimeric 1N11 (mch1N11), ibrutinib (32765) and anti-PD-1 antibody using C57BL/6 mice bearing triple negative E0771 breast tumors. Tumors were staged to an initial volume of ~100mm3and randomized to treatment groups (N=10) with mch1N11 or isotype at 10 mg/kg qw, anti-PD-1 at 2.5 mg/kg qw or ibrunitib 6 mg/kg or vehicle qd x 8. Tumor volumes were measured twice per week to determine tumor growth inhibition (TGI) relative to control treated animals until a maximum volume of 1500-2000mm3. The in vitro sensitivity of E0771 tumor cells to ibrutinib was compared to drug sensitive Jeko-1 lymphoma cells in a 72 hour growth and viability assay.
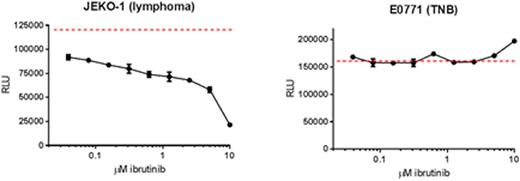
Results: The E0771 cell line is resistant in vitroto 10 mM ibrutinib compared to the drug-sensitive Jeko-1 cell line (Figure 1). Mice bearing E0771 tumors treated with mch1N11, ibrutinib and anti-PD-1 alone had 22.2%, 23.5% and 32.6% TGI respectively. Combination of two agents increased the TGI for mch1N11 and ibrutinib to 30.5%, ibrutinib and anti-PD-1 to 34.5%, mch1N11 and anti-PD-1 to 36.1%. A triple combination therapy had statistically greater TGI compared to control treated mice (59.9%, p = 0.0084) and was greater than single and double combination therapies.
Conclusion:Treatment of solid tumors with a combination of inhibitors that target PS, ITK and the PD-1/PD-L1 axis in the tumor microenvironment provides a novel treatment for solid tumors, including triple negative breast cancer.
Gong:Peregrine Pharmaceuticals, Inc.: Employment. Gray:Peregrine Pharmaceuticals, Inc.: Employment. Hutchins:Peregrine Pharmaceuticals, Inc.: Employment. Freimark:Peregrine Pharmaceuticals, Inc.: Employment.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal