Abstract
Background
Primary CNS lymphoma (PCNSL) is a rare extra nodal non-Hodgkin lymphoma. High-dose methotrexate (HD-MTX) based treatment has improved prognosis, but little is known about the development of quality of life (QoL) during treatment and follow-up from prospective trials.
Methods
We pooled data from three prospective trials including 225 immunocompetent patients with PCNSL (186 [83%] with newly diagnosed and 39 [17%] with relapsed disease) - all received HD-MTX based polychemotherapy with rituximab. QoL was a pre-specified secondary endpoint in all trials and evaluated using the EORTC QLQ-C30 and EORTC QLQ-BN20 questionnaire at start of treatment, after completion of treatment and during follow-up. We used descriptive statistics to summarize QoL over time. To investigate the development of QoL over time adjusted for age and Karnofsky performance status (KPS), we used multivariable linear regression models with a random effect to account for repeat measurements. We also did two separate analyses for patients with newly diagnosed PCNSL and relapsed disease. Multiple imputations were applied for missing values within a questionnaire, but we did not impute values of questionnaires that were missing completely. We herein report results on the global health status (GHS, item 30 of EORTC QLQ-C30, higher values represent better QoL) and the predominant item of the EORTC QLQ-BN20 (future uncertainty, higher values represent worse QoL). A change of 10% on the respective scale was considered as a clinically meaningful difference (Osoba et al, Eur J Cancer. 2005 Jan;41(2):280-7 and Maringwa et al, Ann Oncol. 2011 Sep;22(9):2107-12).
Results
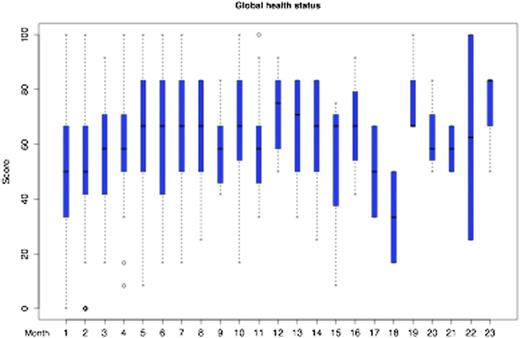
The median age and KPS was 62 years (range 20 to 85) and 80% (30% to 100%), respectively, 119 (52%) were female. Before treatment, the median GHS of the EORTC QLQ-C30 was 50 (interquartile range [IQR] 33 to 67) reflecting substantial impaired QoL. After completion of treatment, the median GHS significantly increased by 17 points to a median of 67 (IQR 50 to 71) (P<0.001), which reflects a clinically meaningful difference. This positive effect was consistent in multivariable analysis (beta coefficient 0.37, p<0.001). Patient with a better KPS before starting treatment had a higher chance that their QoL improved over time (beta coefficient 0.26, p=0.0029). There was no difference regarding GHS between patients with newly diagnosed PCNSL or patients with relapsed disease before starting treatment (median GHS both 50). However, in patients with relapsed disease, numerical increase of GHS over time did not reach statistical significance (beta coefficient 0.36, p=0.2185) likely to limited power in the multivariable analysis. The overall development of GHS over time is shown in Figure 1. The dip at months 17 and 18 area is associated with disease relapse. Regarding the EORTC QLQ-BN20 questionnaire, the predominant issue for patients was future uncertainty (median 42, IQR 17 to 67) before starting treatment, which significantly improved after treatment to 25 (IQR 8 to 33) (p<0.001). In multivariable analysis, future uncertainty also improved significantly in separate analyses for patients with newly diagnosed PCNSL (beta coefficient -0.45, p=0.005) and relapsed disease (beta coefficient -0.89, p=0.0049). The overall development of future uncertainty over time is shown in Figure 2. The increase at months 17 and 18 area is associated with disease relapse.
Conclusions
Patients with newly diagnosed or relapsed PCNSL reported substantial impaired QoL before starting treatment. However, after treatment with HD-MTX based chemotherapy, QoL significantly improved over time and patients were also more confident regarding their future. Results from our analyses provide a reference for future studies on this important issue in the care of patients with PCNSL. At the meeting we will present further analyses and results from all domains of the EORTC QLQ-C30 and EORTC QLQ-BN20 questionnaires.
Global Health Status development from the EORTC QLQ-C30 questionnaire (higher values denote better quality of life)
Global Health Status development from the EORTC QLQ-C30 questionnaire (higher values denote better quality of life)
Future uncertainty from the EORTC QLQ-BN20 questionnaire (lower values denote better quality of life)
Future uncertainty from the EORTC QLQ-BN20 questionnaire (lower values denote better quality of life)
Kasenda:Riemser: Other: Travel Support. Illerhaus:Riemser, Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal