Background
The international prognostic risk score (IPSS) is a validated tool in the prognosis of myelodysplastic syndromes (MDS). Patients who fall into the Int-2 and High categories of the IPSS qualify for treatment with 5-azacitidine, a hypomethylating agent that has been shown to provide a survival advantage with these prognostic risk scores. The IPSS was recently updated, with the newer revised IPSS (IPSS-R) risk score taking into account more cytogenetic data than the IPSS. It has not yet been determined whether the IPSS-R provides an advantage over the IPSS in predicting outcome with 5-azacitidine. The objective of this study was to reclassify a cohort of MDS patients at a single academic center using the IPSS-R, and to determine its ability to predict survival and response to 5-azacitidine.
Methods
This study was approved by the institutional board review. A total of 97 patients with a diagnosis of MDS were treated with 5-azacitidine at the London Health Science Center up until 2015. Both the IPSS and IPSS-R could be calculated for 77 patients. Kaplan-Meier survival curves were constructed for the patients categorized based on the IPSS and IPSS-R. The scores were also compared based on objective response rates (ORR = complete response + partial response + hematologic improvement) as defined by the IWG 2006 criteria that were determined for all patients.
Results
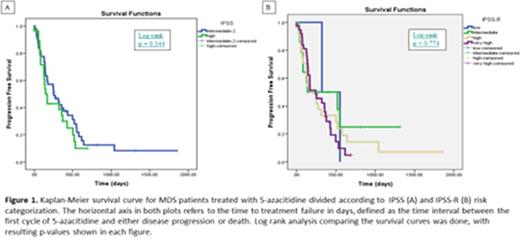
All 77 patients included in the study had qualified for 5-azacitidine treatment according to provincial criteria, and had an IPSS score int-2 or high. Upon reclassification using the IPSS-R, 2/77 patients were scored as low, 14/77 patients were scored as intermediate, 24/77 patients were scored as high, and 37/77 patients were scored as very high risk. Kaplan-Meier survival analysis (Figure 1) indicated there was no statistically significant difference between the IPSS-R intermediate, high, and very high survival curves (log-rank p = 0.774). The objective response rate for all 77 patients treated with 5-azacitidine was 23%, with ORR of 21% for IPSS-R intermediate, 17% for IPSS-R high, and 38% for IPSS-R very high.
Conclusions
The IPSS-R is an alternative prognostic risk scoring system that takes into account additional cytogenetic data compared to the IPSS. In our center the IPSS-R has not resulted in prognostic risk categories that are an improvement over the IPSS in predicting survival in response to 5-azacitidine. The IPSS-R very high risk patients had double the objective response rate to 5-azacitidine compared to the intermediate and high risk groups.
Lazo-Langner:Bayer: Honoraria; Pfizer: Honoraria; Daiichi Sankyo: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal