Abstract
Introduction: There are few reports of standard of care and outcomes in Latin America. The HOLA study is a retrospective chart review of patients with B-cell malignancies in Latin America (LATAM).
Methods: The objective of this registry is to describe patient characteristics, diagnostic and treatment patterns, and clinical outcomes in patients with Chronic Lymphocytic Leukemia (CLL) from a mix of public and private sites in Brazil, Mexico, Chile, Argentina, Colombia, Guatemala, and Panama. We report 472 patients with CLL diagnosed in the period from January 2006 to March 2016.
Results: Median age at diagnosis was 66 years (range 23 - 95). Male gender predominance (53.6 vs 46.4%). Twenty-three percent of the patients (n=103) were dead, median time from diagnosis to death of 2.4 years (range 0-6.6 years). Seventeen percent (n=19) died before frontline treatment, 35%(39) after first-line treatment, 19%(21) in second-line, 27%(30) at or after third-line treatment.
Anemia reported on 34%. Of the 34% (n=162) patients with COOMBS test, it was positive on 13%.
Frequent comorbidities were high blood pressure (46%), heart disease (17%) and diabetes (15%).
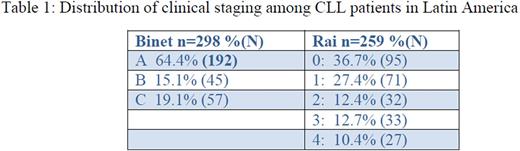
Binet status reported on 63% of patients, Rai status on 55%. (Table 1)
Flow cytometry missing on 27% of patients (not performed/missing report). Diagnostic markers were positive as follows: CD23, 94% and CD5, 92%. Prognostic markers: 52% tested for CD38 and 10% ZAP 70, positivity on 24% and 26% of tested patients, respectively. Cytogenetic/FISH test performed on 21% (102), del(17p) was present on 7.4% of tested patients at diagnosis.
Seventy two per cent of patients were considered for watch- and-wait approach. Median time from diagnosis to treatment initiation was 113 days (range 1 -2808).
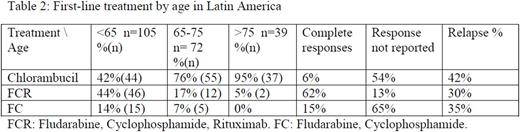
Sixty one per cent (288/472) receive front line treatment at any time. Frequent reasons to start treatment were B symptoms (25%), anemia (21%) and lymphocyte doubling time <6 months (17%). (Table 2)
Response was assessed by clinical exam and blood count in 95% of patients, CT scan 24 %, abdominal ultrasonography 12%.
Twenty-eight per cent of patients (135) relapsed and received second-line treatment. The most frequent second-line treatments were Chlorambucil (Chl) 37%; Fludarabine + Cyclophosphamide (FC) 16%, Cyclophosphamide + Vincristine + Prednisone (CVP) 9%. Clinical complete responses were as follows: FC = 4.5%, FCR= 25%.
Sixty-five out of 472 patients (14%) received third-line treatment. Most frequently used treatments at this line were: Chlorambucil 23%, FC 20%, CVP 13%, FCR 9.2%, BR 4.6 %, and other chemotherapies 4.6%.
Only 7% of patients in the registry relapsed and received fourth-line treatment.
Conclusions:
HOLA registry describes patterns of demographics, diagnosis and treatments in the real world in Latin America. CLL diagnosis in Latin America is made based on flow cytometry for the majority of the patients. Cytogenetic and FISH test are scarcely performed.
For the deceased patients, median time from diagnosis to death was 2.4 years. This could be related to the high risk, but since prognostic tests were not always present this merits further investigation.
Watch-and-wait was the most frequent approach. Time elapsed from diagnosis to treatment initiation was less than a year. Lymphocyte doubling time as reason to start treatment was frequent.
Chlorambucil remains the most frequently used treatment for elderly in newly diagnosed and relapsed /refractory patients, use is also frequent in the young/fit patient population. Access barriers to innovative drugs and monoclonal antibodies could explain this situation.
Chiattone:Janssen: Membership on an entity's Board of Directors or advisory committees; roche: Membership on an entity's Board of Directors or advisory committees; abbvie: Membership on an entity's Board of Directors or advisory committees. Gomez-Almaguer:Bristol: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Pavlovsky:Janssen: Speakers Bureau; Novartis: Speakers Bureau; Bristol Myers Squib: Speakers Bureau. Tuna-Aguilar:Janssen: Speakers Bureau. Farias:Janssen: Consultancy. Galvez:Novartis: Consultancy. Santos:Janssen: Employment. De La Mora Estrada:Janssen: Employment, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal