Abstract
Introduction: Idelalisib (IDELA) is a targeted PI3Kd inhibitor approved as monotherapy in relapsed follicular lymphoma and in combination with rituximab in relapsed chronic lymphocytic leukemia (CLL). Increased rates of adverse events (AEs) were recently observed in the IDELA vs placebo arms of randomized controlled trials (RCT) evaluating IDELA added to standard therapies in front-line CLL and early-line indolent non-Hodgkin lymphoma (iNHL). AEs leading to death were mainly infectious and included pneumocystis jirovecii pneumonia (PJP) and cytomegalovirus (CMV). This analysis across trials in the relapsed population evaluated whether quantitative changes in lymphocyte subsets may have contributed to these AEs.
Methods: Peripheral blood immunophenotypic data available for analysis from patients (pts) (n = 1,480) treated in 5 IDELA RCTs were analyzed. Three studies (n = 787) included pts with relapsed CLL (NCT01569295: IDELA + bendamustine-rituximab [BR] vs placebo + BR; NCT01539512: IDELA + R vs placebo + R and NCT0165902: IDELA + ofatumumab [O] vs placebo + O) and 2 studies (n = 693) included R/R iNHL pts (NCT01732913: IDELA + R vs placebo + R and NCT01732926: IDELA +BR vs placebo + BR). Absolute numbers of T (CD4+ and CD8+), B (CD19+) and NK (CD16+/CD56+) cells were analyzed longitudinally in both IDELA and placebo pts across the 5 studies. Lymphocyte subsets were analyzed separately in those who died and then correlated with specific grade ≥3 AEs including infections, febrile neutropenia, and respiratory (acute respiratory failure, pneumonitis) events. Analysis was conducted within individual study and for all studies combined. Of note, samples were collected more frequently during the first 6 months (during combination therapy) and collection times varied among the 5 studies.
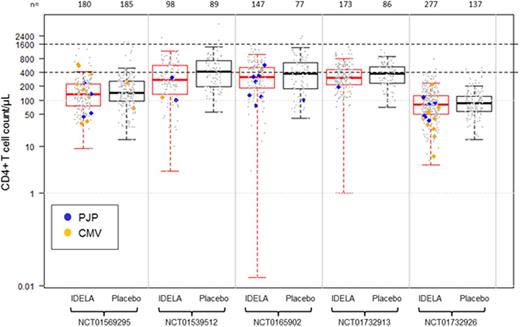
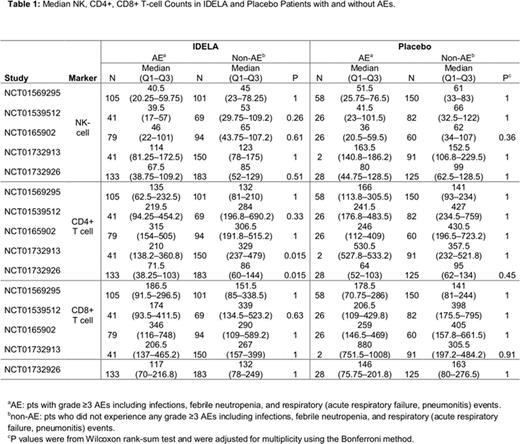
Results: There was no specific trend noted with the CD8+ T-cells between treatment groups across the studies. Generally, NK-cells were decreased to a similar degree in both IDELA and placebo pts at weeks 10 to 12 with recovery starting around week 24. There were no differences in median NK- and CD8+ T-cell counts between pts with grade ≥3 AEs and no AEs within either group. In both BR trials, CD4+ T-cells nadir to <200 cells/µl occurred at week 22 in both groups. Recovery of CD4 to ≥200 cells/µL occurred at week 30 in CLL pts and at week 72 in iNHL pts. Median CD4+ T-cells in pts on the BR trials were lower in groups both with and without AEs, compared with non-BR trials (Table 1). There were a total of 31 cases of PJP (13 in the BR trials) and 32 cases of CMV infection (28 in the BR trials). Analysis of PJP and CMV pts with available immunophenotypic data (n = 46) revealed that 33 pts had CD4 <200 cells/µL; 31 of these were treated with IDELA plus combination therapy (Figure 1). Finally, while there were more grade ≥3 AEs within IDELA arms, these did not occur at any specific CD4 level and, in fact, grade ≥3 AEs were noted even in pts with CD4 >900 cells/µL.
Conclusion: Within 5 RCTs evaluating IDELA vs placebo in combination with an anti-CD20 mAb or BR in R/R CLL or iNHL, there was no correlation between grade ≥3 AEs and NK- or CD8+ T-cell counts. Median CD4+ T-cells in pts on the BR trials were lower in both groups in those with and without AEs, compared with non-BR trials. In addition, pts with PJP and CMV infections were noted to have CD4+ T-cells <200 cells/ µL, and this was more common in IDELA-treated patients, especially when combined with BR, suggesting that the lymphosuppressive effect of IDELA may augment the myelosuppressive effect of bendamustine. While this current study involves the quantitative analysis of various immune cell subsets, it may be the qualitative function of these cells that contributed to infections. Assays evaluating the qualitative function of these cells are being investigated. All IDELA trials have been amended to include PJP prophylaxis and CMV monitoring.
Sharman:Gilead Sciences, Inc.: Honoraria, Research Funding. Salles:Mundipharma: Honoraria; Amgen: Consultancy, Honoraria; Gilead: Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria, Research Funding. Jurczak:Celltrion, Inc: Research Funding; Janssen: Research Funding; Gilead Sciences: Research Funding; Acerta: Research Funding; Bayer: Research Funding. Jones:AbbVie: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Gilead Sciences: Consultancy, Research Funding; PCYC: Consultancy, Research Funding; Janssen: Consultancy, Research Funding. Owen:Janssen: Honoraria; Gilead: Honoraria, Research Funding; Pharmacyclics: Research Funding; Celgene: Honoraria, Research Funding; Abbvie: Honoraria; Lundbeck: Honoraria, Research Funding; Novartis: Honoraria; Roche: Honoraria, Research Funding. Munugalavadla:Gilead Sciences: Employment, Equity Ownership. Dreiling:Gilead Sciences: Employment, Equity Ownership. Xiao:Gilead Sciences: Employment, Equity Ownership. Rao:Gilead Sciences: Employment, Equity Ownership. Flinn:Janssen: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Gilead Sciences: Research Funding; ARIAD: Research Funding; RainTree Oncology Services: Equity Ownership. O'Brien:Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal