Abstract
Introduction: Multiple myeloma (MM), the second most frequent hematologic malignancy, is characterized with clonal proliferation of malignant plasma cells in the bone marrow, and usually monoclonal protein in the blood and/or urine. Its clinical manifestations are associated with end-organ damage consisting of anaemia, renal insufficiency, bone lesions and hypercalcaemia. Long non-coding RNAs (lncRNAs) represent more than half of the mammalian noncoding transcriptome and are involved in many biological processes, including transcription regulation, competition with other linear RNAs and involvement in tumorigenesis or oncogenic pathways. Accumulating studies have revealed the important role of lncRNAs in the hematological tumors, especially MM. In order to investigate the further relationship between MM and lncRNAs, we performed global lncRNA profiling in both incipient MM patients and iron deficiency anemia (IDA) patients. We assumed that the bunch of deregulated lncRNAs in MM patients would broaden current understanding of progression of MM, and present a new approach to novel therapeutic targets.
Methods: Bone marrow mononuclear cells were obtained from five MM patients with normal karyotype at the Department of Hematology, Second Affiliated Hospital, Xi'an Jiaotong University, in 2016. Additionally, bone marrow samples from five patients with IDA were used as controls. One sample in five MM patients was rejected because of its disqualification in clustering analysis. Arraystar Human LncRNA Array V4.0 was used to profile expression of lncRNAs, which was performed by KangChen Bio-tech (Shanghai, China). The array detects a total of 40,173 lncRNAs probes and an entire collection of 20,730 protein coding mRNAs. Gene Ontology (GO) analysis (http://david.abcc.ncifcrf.gov/summary.jsp) was used to analyze differentially expressed transcripts. Pathway analysis of differentially expressed transcripts were accomplished using Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://www.genome.jp/kegg).
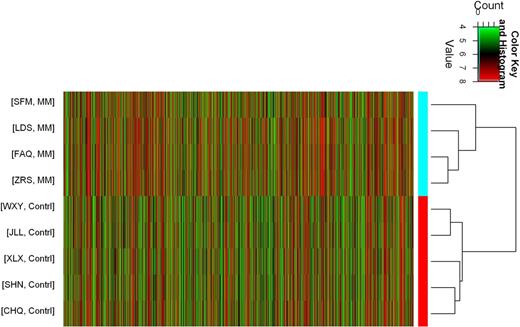
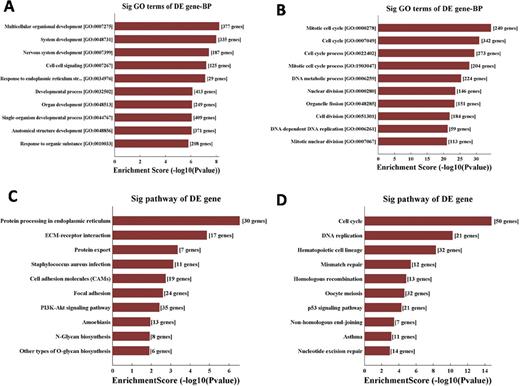
Results: Bioinformatic analysis of the lncRNA expression identified a total of 2445 lncRNAs were upregulated and 1519 lncRNAs were downregulated in four MM samples, among them, 199 lncRNAs were significantly upregulated and 27 lncRNAs were notably downregulated more than 10-fold in MM samples compared with IDA (Figure 1). GO analysis by Database for Annotation, Visualization and Integrated Discovery (DAVID ) showed that the top ten enriched biological process (BP) by upregulated transcripts were involved in multicellular organismal development and cell-cell adhesion, while downregulated transcripts were dragged in mitotic cell cycle and cell cycle. KEGG pathway analysis of the top ten enriched pathways included ECM-receptor interaction and protein processing in endoplasmic reticulum in upregulated transcript, while cell cycle and DNA replication were the top two enriched pathways in downregulated transcript (Figure 2). Among them, mitotic cell cycle was the most enriched pathway with p-value 3.86E-35. These results suggested that the dysregulated lncRNAs were related to MM cells adhesion and proliferation or differentiation closely.
Conclusions: We have identified a group of dysregulated lncRNAs and mRNAs in bone marrow samples obtained from MM patients versus IDA controls. These lncRNAs participate in MM cell growth, migration and invasion by regulating cell adhesion and cell cycle, playing an important role in the development and progression of MM. Further investigation is still required to detect the underlying functions of these lncRNAs in MM pathogenesis and whether these lncRNAs may serve as new diagnostic biomarkers and therapeutic targets for MM.
Clustering analysis of four mulitiple myeloma (MM) samples and five iron deficiency anemia (IDA) controls.
Clustering analysis of four mulitiple myeloma (MM) samples and five iron deficiency anemia (IDA) controls.
Gene Ontology(GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of dysregulated transcripts. (A) The top ten enriched biological process (BP) by upregulated transcripts. (B) The top ten enriched BP by downregulated transcripts. (C) The top ten enriched pathways in upregulated transcripts. (D) The top ten enriched pathways in downregulated transcripts.
Gene Ontology(GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of dysregulated transcripts. (A) The top ten enriched biological process (BP) by upregulated transcripts. (B) The top ten enriched BP by downregulated transcripts. (C) The top ten enriched pathways in upregulated transcripts. (D) The top ten enriched pathways in downregulated transcripts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal