Abstract
Introduction: Lenalidomide (Len) and dexamethasone (dex) has proven to be a highly effective treatment for multiple myeloma (MM) and serves as the basis of other combinations in relapsed disease. However, nearly 1/3 of myeloma patients have renal insufficiency, and the optimal use of Len in this population is not well known. We undertook this study in 3 cohorts of pts: Group A had creatinine clearance (CrCl) of 30-60 ml/min, Group B with CrCl < 30 ml/min, and Group C, with CrCl < 30 ml/min and requiring dialysis. The Phase I component of the trial has been previously reported (ASCO 2014) demonstrating that full dose Len (25mg daily 21/28 days) can be given to all patients despite renal insufficiency, even when on dialysis. We now present data on response to treatment.
Methods: In the Phase 2 component accrual was slow and the trial was terminated. However, prior to termination a modified exploratory analysis plan was established, including all 34 patients treated at the recommended phase 2 dose. This plan would declare the treatment worthy of further study if 18 or more of the 34 patients responded to treatment. This design had 84% power to distinguish a response rate of 60% from a null rate of 40%, using a 1-sided test with 10% type I error.
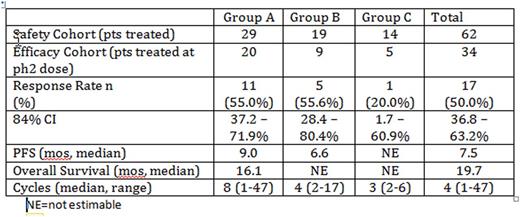
Results: The Phase 2 efficacy cohort accrued 34 patients, consisting of 20 patients from Group A, 9 patients from Group B, and 5 patients from Group C. Response of PR or greater was seen in 17 patients, resulting in a 50% overall response rate (CI 37-63%). Treatment was well tolerated with expected adverse events; the most common adverse events were anemia, diarrhea, and fatigue. Eleven episodes of Grade 3 and one Grade 4 pneumonia were reported for 10 patients across all cohorts and dose levels; 3 were considered possibly related to treatment. Seventeen patients have remained on treatment for more than 12 cycles. In the exploratory analyses, median PFS was 7.5 months (95% CI, 3.6 - 19.7 months) and median OS was 19.7 months (95% CI, 10.4 - 42.5 months).
Conclusions: Len Dex can be safely administered to patients with impaired renal function at full dose of 25mg daily 21/28 days, and is associated with a 50% response rate. Further investigation into other combinations with Len Dex in patients with renal impairment should be considered.
Mikhael:Onyx: Research Funding; Sanofi: Research Funding; Abbvie: Research Funding; Celgene: Research Funding. Lonial:Merck: Consultancy; Novartis: Consultancy; Onyx: Consultancy; BMS: Consultancy; Onyx: Consultancy; Celgene: Consultancy; BMS: Consultancy; Novartis: Consultancy; Celgene: Consultancy; Janssen: Consultancy; Millenium: Consultancy; Janssen: Consultancy. Weiss:Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal