Abstract
Introduction: Previously reported results from this ongoing study showed clinical activity for polatuzumab vedotin (PoV), an antibody drug conjugate (ADC) containing the anti‐mitotic MMAE targeting CD79b, at a dose of 2.4 mg/kg, in combination with rituximab (R) in relapsed/refractory (r/r) diffuse large B‐cell lymphoma (DLBCL) and follicular lymphoma (FL) when patients (pts) were treated until progression (Morschhauser ASH 2014, Advani ASCO 2015). Obinutuzumab (G) is a next generation antiCD20 mAb with enhanced antibody-dependent cellular cytotoxicity.and direct cell death. We report here the safety and efficacy results for this Phase Ib/II study of PoV at 1.8 mg/kg combined with G treated for up to 8 cycles in r/r DLBCL or FL (ClinicalTrials.gov NCT01691898).
Methods: This multicenter, open-label Ph Ib/II study evaluated the combination of PoV with G in pts with r/r DLBCL or FL. Primary objective was to assess the anti-tumor activity of PoV + G; secondary endpoints included safety and tolerability and pharmacokinetics. Adult pts (≥18 years) were treated with PoV 1.8 mg/kg intravenously with G every 21 days for a total of 8 cycles. Assessments for anti-lymphoma activity were performed following 4 cycles of study treatment (tx) and at the end of study tx (EOT) by Lugano 2014 criteria.
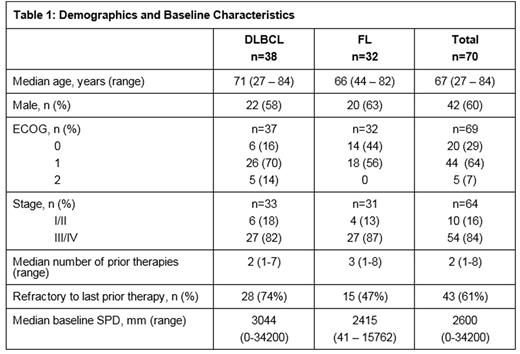
Patients: As of Apr 5, 2016, 70 (38 DLBCL, 32 FL) pts were evaluable for safety. The demographic and baseline characteristics are included in Table 1.
Results: Of 70 safety-evaluable patients, 49 (70%) experienced a treatment-related adverse event (AE) of which most were mild (Gr 1/2; 34 of 49). The most common AEs in >20% of pts were Gr 1-3 fatigue (43%), Gr 1-2 diarrhea (34%), Gr 1-3 nausea (30%), Gr 1-2 constipation (21%) and Gr 1 headache (20%).
Among the 70 pts who received at least 1 dose of tx, 46% (32/70) pts had Grade 3/4 AEs and 29% (13 DLBCL, 7 FL) had serious AEs (SAEs). The most common Grade 3/4 AE was neutropenia (13%) and the most common SAE occurring in >10% was infections (15%) including pneumonia (n=3), influenza, urinary tract infection (n=2 each), sepsis, sinusitis, and respiratory tract infection (n=1 each). Four pts (2 DLBCL, 2 FL) had Gr 2 peripheral neuropathy (PN, n=2) and peripheral sensory neuropathy (n=2). Onset of PN occurred after cycle 2 and cycle 3 in the DLBCL pts and after cycle 5 in both FL pts. All 4 pts required dose reduction of PoV (1.8 to 1.4 mg/kg). Five pts discontinued PoV due to AE (3 DLBCL, 2 FL), including Gr 3 GI perforation (n=1), Gr 3 malignant neoplasm (n=1), and Gr 3 delirium and urinary tract infection (n=1) in pts with DLBCL, and Gr 5 pneumonia and Gr 2 elevated bilirubin (n=1, each) in FL pts. Eight deaths (1 due to pneumonia in a pt with FL and 7 due to progressive disease in pts with DLBCL) were reported.
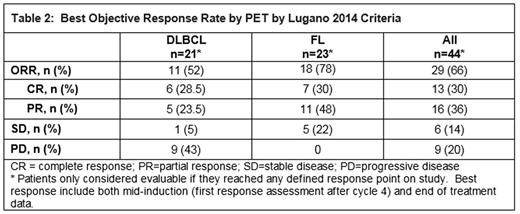
The median number of treatment cycles received was 2 (0-8) and 5 (0-8) for the pts with DLBCL and FL, respectively. Best objective response rate by PET was available for 44 evaluable (21 DLBCL, 23 FL) pts. The regimen was active in heavily pre-treated pts with an ORR of 78% for FL and 52% for DLBCL (see Table 2).
Updated safety and efficacy data will be presented.
Conclusions: Early results from this ongoing study show that this novel combination of PoV, at 1.8 mg/kg with fixed duration (≤ 8 cycles), plus G has an acceptable safety profile with evidence of clinical activity in r/r DLBCL or FL pts who were heavily pretreated or refractory to the last prior regimen.
Brunvand:Millennium / Takeda: Speakers Bureau; Celgene: Speakers Bureau; Genentech: Speakers Bureau. Chen:Seattle Genetics: Consultancy. Press:Roche / Genentech: Consultancy, Research Funding. Chiappella:Pfizer: Speakers Bureau; Celgene: Speakers Bureau; Roche: Speakers Bureau; Janssen-Cilag: Speakers Bureau; Teva: Speakers Bureau; Amgen: Speakers Bureau. Diefenbach:Genentech: Honoraria, Research Funding. Jones:Genentech, Inc.: Employment. Hirata:Genentech: Employment. Flinn:Janssen: Research Funding; Gilead Sciences: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; ARIAD: Research Funding; RainTree Oncology Services: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal