Abstract
Introduction:
In chronic lymphocytic leukemia (CLL), improved anti-CD20 antibodies such as obinutuzumab (GA101) and targeted drugs, such as ibrutinib, show very good efficacy and tolerability.
With the CLL2-BIG trial, the German CLL Study Group designed a regimen composed of obinutuzumab and ibrutinib for induction and maintenance therapy. Patients with high tumor burden receive bendamustine as debulking resulting in the proposed "sequential triple-T" concept [Hallek M., Blood 2013] of a tailored and targeted treatment aiming at total eradication of minimal residual disease (MRD). Here we present the results of the primary endpoint analysis of the trial.
Methods:
This prospective, open-label, multicenter phase-II trial investigates the efficacy and safety of a novel regimen for physically fit and unfit CLL patients (pts) requiring treatment, irrespective of high-risk genetics. 62 pts were to be recruited according to a predefined allocation for the two strata of first-line and relapse/refractory treatment.
Six cycles of induction treatment with obinutuzumab and ibrutinib were administered followed by maintenance therapy with continuous ibrutinib and obinutuzumab every three months until achievement of an MRD-negative complete remission or up to 24 months. Pts with an absolute lymphocyte count ≥ 25.000/µl and/or lymph nodes ≥ 5cm received two cycles of bendamustine before start of induction.
The primary endpoint is overall response rate (ORR) three month after the start of last induction cycle administered; secondary endpoints include ORR after debulking, MRD evaluations and safety parameters (graded per the NCI CTCAE v.4 criteria).
Results:
66 pts were enrolled between January and August 2015. Five pts completed less than two cycles of induction and were therefore excluded from the full analysis set as defined by protocol. The current analysis includes 58 pts; an update including all 61 pts will be available for presentation at the meeting.
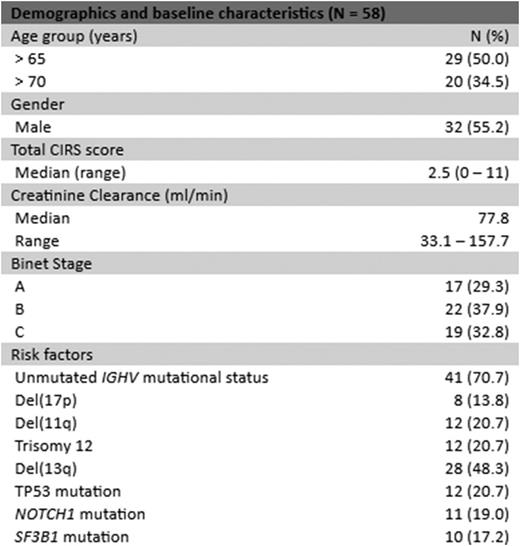
The median age was 65.5 (range 36-83) years and the median time since initial diagnosis was 56.2 (2.1 - 222.8) months. 27 pts were treatment-naïve and 31 relapsed/refractory with a median of one prior treatment line (1-5). Baseline characteristics are summarized in table 1.
41 pts (71%) received bendamustine debulking, of these 27 pts (66%) responded; five pts (12%) achieved clinical complete remission (CR), 3 pts (7%) clinical CR with incomplete recovery of the bone marrow (CRi) and 19 pts (46%) partial remission (PR).
57 pts completed six cycles of induction treatment. One patient died after the fifth course due to grade 5 duodenitis related to study therapy. The combination showed promising efficacy with an ORR of 100% by investigator assessment at the end of induction. Statistically, the primary endpoint was met and the null hypothesis could be rejected. Response rates are presented in table 2.
27 pts (47%) achieved MRD negativity (< 10-4 by flow cytometry) in peripheral blood (pB) at the end of induction. An intermediate MRD-status (≥ 10-4 and < 10-2) was found in 15 pts (26%) whereas 13 pts (22%) were MRD positive (≥ 10-2). In three pts (5%) no MRD sample was available. So far, 38 pts (65%) received at least one dose of maintenance treatment; one patient already stopped treatment due to MRD negativity as defined per protocol.
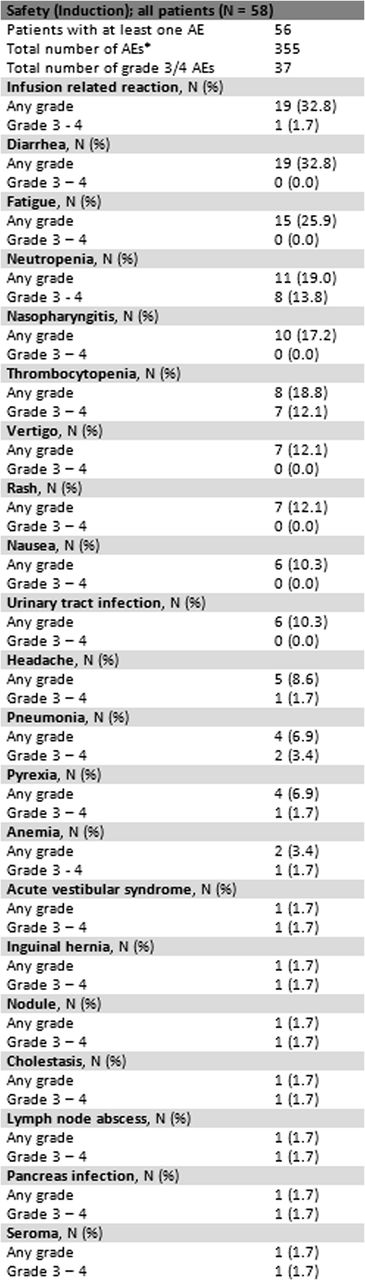
CTC Grade 3-4 adverse events occurred in 19 pts (33%) during induction therapy. The most common toxicities observed are shown in table 3.
*The table includes all grade 3-4 AEs regardless of frequency and AEs of any grade observed in at least six patients.
Conclusion:
With an ORR of 100% and an MRD negativity rate of 47% in the pB the BIG-regimen showed a very good efficacy in a heterogeneous study population. No major toxicity occurred so far.
Demographics and baseline characteristics
Response rates
Safety
von Tresckow:Janssen-Cilag: Honoraria, Other: Travel grants, Research Funding; Celgene: Other: Travel grants; Hoffmann-LaRoche: Other: Travel grants, Research Funding. Cramer:Astellas: Other: Travel grants; Janssen-Cilag: Honoraria, Other: Travel grants, Research Funding; GlaxoSmithKline/Novartis: Research Funding; Gilead: Other: Travel grants, Research Funding; Hoffmann-LaRoche: Honoraria, Other: Travel grants, Research Funding; Mundipharma: Other: Travel grants. Bahlo:F. Hoffman-La Roche: Honoraria, Other: Travel grant. Engelke:Hoffmann-LaRoche: Other: Travel grants. Langerbeins:Hoffmann-LaRoche: Honoraria, Other: Travel grants, Research Funding; Janssen-Cilag: Honoraria, Other: Travel grants, Research Funding; Mundipharma: Honoraria, Other: Travel grants, Research Funding. Fink:Mundipharma: Other: Travel grants; Celgene: Other: Travel grants, Research Funding; AbbVie: Other: Travel grants; Hoffmann-LaRoche: Other: Travel grants. Illmer:Hoffmann-LaRoche: Honoraria, Other: travel grants. Ritgen:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Fischer:Hoffmann-LaRoche: Other: Travel grants. Wendtner:Mundipharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding; GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding; Hoffmann-LaRoche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Research Funding. Stilgenbauer:Janssen: Consultancy, Honoraria, Other: Travel grants , Research Funding; Hoffmann-La Roche: Consultancy, Honoraria, Other: Travel grants , Research Funding; Pharmacyclics: Consultancy, Honoraria, Other: Travel grants , Research Funding; Amgen: Consultancy, Honoraria, Other: Travel grants, Research Funding; GSK: Consultancy, Honoraria, Other: Travel grants , Research Funding; Mundipharma: Consultancy, Honoraria, Other: Travel grants , Research Funding; Genentech: Consultancy, Honoraria, Other: Travel grants , Research Funding; Boehringer Ingelheim: Consultancy, Honoraria, Other: Travel grants , Research Funding; Celgene: Consultancy, Honoraria, Other: Travel grants , Research Funding; Novartis: Consultancy, Honoraria, Other: Travel grants , Research Funding; Sanofi: Consultancy, Honoraria, Other: Travel grants , Research Funding; Gilead: Consultancy, Honoraria, Other: Travel grants , Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel grants, Research Funding; Genzyme: Consultancy, Honoraria, Other: Travel grants , Research Funding. Böttcher:AbbVie: Honoraria, Research Funding; Hoffmann-LaRoche: Honoraria, Other: Travel grants, Research Funding; Celgene: Research Funding. Eichhorst:Abbvie: Consultancy; Mundipharma: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau. Hallek:Amgen: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; Mundipharma: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; F. Hoffmann-LaRoche: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: travel support, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal