Abstract
Myeloablative busulfan with cyclophosphamide (BuCy) has been the standard conditioning regimen for children with myeloid malignancies and certain non-malignant conditions. While effective, this regimen has historically high rates of regimen-related toxicities such as sinusoidal obstruction syndrome (SOS; 20-50%) and hemorrhagic cystitis (5%-15%). Substituting fludarabine for cyclophosphamide (BuFlu) has led to less toxicity and better outcomes in adult patients, prompting many pediatric centers to adopt this regimen despite sparse pediatric medical literature. We utilized the CIBMTR database to compare toxicity and outcomes of BuCy and BuFlu in pediatric patients.
Methods: We identified 1781 patients aged 0-18 receiving a first allogeneic transplant with myeloablative BuCy (N=1400) or BuFlu (N=381) from 2008-2014. We excluded recipients of cord blood, T-cell depleted grafts and haplo-identical donors. The completeness index of follow up was 93% at 2 years. Transplant-related mortality (TRM), relapse and disease free survival (DFS) for patients with malignant diseases, and overall survival (OS) for all patients were compared between regimens. For a subset of patients with available comprehensive reporting (N=528; BuCy N=364, BuFlu N=164), we compared neutrophil and platelet engraftment, SOS, non-infectious hemorrhagic cystitis and pulmonary toxicity, graft failure, infections, acute and chronic GVHD between regimens.
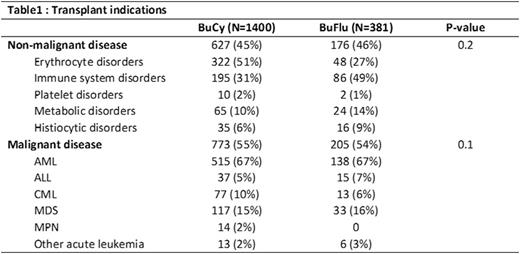
Results: For patients with non-malignant diseases (BuCy N=627, BuFlu N=176; Table 1), the BuFlu group had a higher proportion of patients with HCT-comorbidity index (HCT-CI) ≥3 and unrelated donors with single HLA-antigen mismatches. With BuCy, SOS and hemorrhagic cystitis occurred at historical rates and more frequently than BuFlu (16% vs. 7%, p=0.04, and 9% vs. 2%, p=0.04). BuFlu recipients experienced better platelet engraftment by day 28 (40% vs. 59%, p=0.005). There was no difference in graft failure, neutrophil engraftment, infections or GVHD (all p>0.05). There was no difference in 2-yr OS (p=0.8). On multivariate analysis, conditioning regimen had no impact on survival (p=0.5).
For patients with malignant diseases (BuCy N=773; BuFlu N=205; Table 1), the BuFlu group had a higher proportion of patients with HCT CI ≥ 3, unrelated donors with single HLA-antigen mismatches, more frequent use of peripheral blood stem cells (PBSC) and ATG. BuFlu was associated with better day 28 neutrophil (91% vs 94%, p=0.002) and platelet engraftment (43% vs 67%, p<0.001), but no differences in engraftment at day 100. There were no differences in SOS (15% vs 13%, p=0.66), hemorrhagic cystitis, (8% vs 7%, p=0.75) pulmonary toxicity, infections and GVHD between regimens. Univariate analysis demonstrated inferior 2-year DFS (62% vs. 56%, p=0.03) and OS (71% vs. 61%, p=0.001) with BuFlu, but no significant differences in TRM or relapse. Multivariate analysis including only risk factors that were significant on multivariate analysis demonstrated higher mortality attributable to BuFlu (HR 1.4, p<0.008; Table 2), but not DFS.
The most common indication for busulfan-based conditioning in children is acute myeloid leukemia (AML) in complete remission (CR). When analyzing the subset of patients with AML in CR (BuCy N=462, BuFlu N=118), there was no difference in SOS, hemorrhagic cystitis, pulmonary toxicity, acute or chronic GVHD, TRM, relapse, DFS or OS.
Conclusions: The use of BuFlu in lieu of BuCy leads to faster engraftment and appeared to reduce the incidence of SOS and non-infectious hemorrhagic cystitis in patients with non-malignant diseases. Other outcomes appear comparable between regimens for patients transplanted for non-malignant diseases. Patients receiving BuFlu for malignancy experienced worse overall survival. These differences in survival were not evident for patients with AML in CR, although numbers were lower. BuFlu recipients were in general more infirm, received alternative donor grafts, PBSC or ATG more often, reflecting that the selection of conditioning was linked to specific transplant scenarios. Despite adjusting for known factors, isolating the sole impact of conditioning on survival is difficult. These findings require confirmation on prospective, randomized trials.
Nishihori:Novartis: Research Funding; Signal Genetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal