Abstract
Background: Tyrosine kinase inhibitors (TKIs) are able to induce, in some chronic myeloid leukemia (CML) patients, long-term undetectable molecular disease (UMD). Several studies have now demonstrated that TKIs could be safely discontinued in those patients previously treated with imatinib (STIM, TWISTER, EUROSKI) and more recently with nilotinib and dasatinib (STOP 2G-TKI). All these studies show a Treatment-Free Remission (TFR) rate reaching ~50%. However, a major issue needs to be resolved for the ~50% of patients that fail such TFR strategies.
Methods: We have previously reported the possibility of a second imatinib discontinuation in 16 patients who obtained a second UMD state according to the STIM criteria (RE-STIM observational study, Legros et al. Blood 2012). Here, we report a larger cohort of patients who attempt twice TKI-discontinuations with enlarged inclusion criteria: Adults CML patients without prior allogeneic transplantation or progression to advanced phase CML undergoing a 2nd attempt of TKI discontinuation for sustained deep molecular response after a 1st failure. All patients were followed in CML reference centers and according to the EUTOS-ELN accreditation criteria for BCR-ABL assessments with minimal numbers of 32,000 ABL copies/sample.
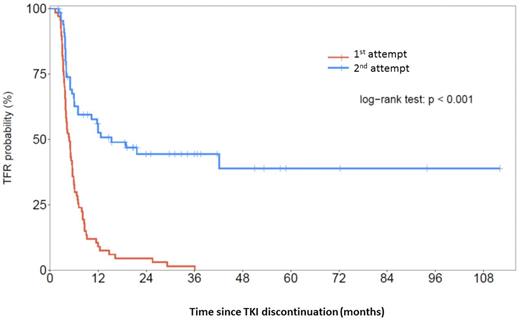
Results: At the time of analysis (1st July 2016), 67 patients (median age: 51 years (range: 25-80 years)) were included. At CML diagnosis, 64 patients were in chronic phase (CP) and 3 patients in accelerated phase (AP). The Sokal risk and the EUTOS long-term survival scores (ELTS) were respectively low in 47% and 68%, intermediate in 36% and 16%, high in 11% and 2% and unknown in 6% and 14% of patients. All patients were treated initially with imatinib and 16% of patients switch to nilotinib (6/11) or to dasatinib (5/11) for intolerance/resistance reasons prior to the 1st TKI discontinuation. The median time on TKI prior to the 1st discontinuation was 63 months (range: 30-146) and the median duration of 1st CMR was 35 months (range: 20-85). The 1st molecular relapse occurred with a median of 2.5 months (range: 0-22) and the second UMD after TKI re-challenge was obtained with a median of 4.4 months (0-40). The reason of the TKI re-challenge was loss of UMD in 43%, loss of MMR in 55% and unknown in 1%. The TKI re-challenge (imatinib 73%, nilotinib 16%, dasatinib 11%) was then administered during a median of 31 months (range: 9-72 months) before the 2nd attempt of discontinuation. At 2nd TKI cessation, 85% of patients were in UMD, 3% in MR4.5, 6 % in MR4, 3% in MMR and 3% unknown. Thirty out of sixty-eight (44%) patients remained treatment-free after a median follow-up of 21.5 months (1-106), see figure. Similarly to 1st attempts, the majority of loss of MMR occurred during the first 6-12 months in this 2nd attempt cohort. Gender, age, disease phase, prognosis scores, prior interferon exposure, initial TKI type, and duration of UMD were not found to have any impact on the outcome after the 2nd attempt in a multivariate analysis. In contrast, a longer time to obtain the first UMD before the 1st attempt was associated with a significantly lower molecular disease-free survival rate after the 2nd discontinuation (p = 0.048). All patients are alive at last follow-up except one who died from an unrelated CML reason (heart attack under imatinib).
Conclusion: TKIs could safely and successfully be discontinued a second time in CML pts despite a 1st failure.
Nicolini:BMS: Consultancy, Honoraria; Ariad pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Etienne:BMS: Speakers Bureau; Pfizer: Speakers Bureau; ARIAD: Speakers Bureau; novartis: Consultancy, Speakers Bureau. Huguet:Pfizer, Novartis, BMS, Ariad, Jazz, Amgen: Membership on an entity's Board of Directors or advisory committees. Guerci-Bresler:Pfizer: Consultancy; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; ARIAD: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Mahon:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ARIAD: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; BMS: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal