Abstract
Despite the increasing availability of targeted therapies for myeloproliferative neoplasms (MPNs), there remains a subset of these patients that transform to secondary acute myeloid leukemia (sAML). MPN patients who develop sAML have a dismal outcome, with a median survival of six months. The mechanisms and pathways that contribute to transformation from MPN to sAML have not been well delineated. The most commonly mutated genes found in the MPN initiating clones include JAK2, MPL and CALR. Transformation to sAML however requires acquisition of additional co-operating mutations such as TET2, IDH1/2, and NRAS. Recent genome sequencing studies identified deletions of JARID2, a gene associated with the Polycomb Repressive Complex 2 (PRC2) involved in implementing global H3K27me3 in post-MPN sAML. Mutations in JARID2 are found only in the blast phase of MPNs, but not in chronic phase of the disease. This data suggests that a JARID2 deletion could be a sAML-specific transforming event by acting as a tumor suppressor in HSCs.
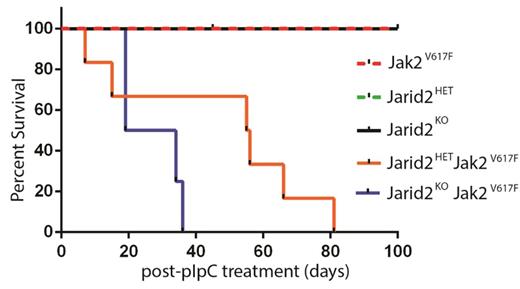
To investigate the role of Jarid2 as a tumor suppressor, we utilized an inducible mouse model of the prototypical MPN driver mutation Jak2V617F. We have established our model system by crossing Mx1-CRE:Jarid2fl/fl (Jarid2KO) or Mx1-CRE:Jarid2fl/+ (Jarid2HET) with JAK2V617F mice to generate a Mx1-CRE:Jarid2fl/fl Jak2V617F/+ or Mx1-CRE: Jarid2fl/+Jak2V617F/+ strain. Mx1-CRE mediates both activation of Jak2V617Fand deletion of Jarid2 simultaneously in adult hematopoietic compartment upon injection of the double-stranded RNA analog polyinosinic:polycytidylic acid (pIpC). In all cases, the absence of Jarid2 in Jak2V617F/+ background accelerated MPN progression, characterized by elevated hemoglobin and hematocrit, increased red blood cells, leukocytosis, thrombocytosis, and splenomegaly compared to the control groups. Median survival of Jarid2KO-Jak2V617F/+ and Jarid2HET-Jak2V617F/+ strains also revealed a dose-dependence of Jarid2 on life expectancy with a median of 27 and 56 days post pIpC treatment, respectively (Figure 1). Together, these data suggest that loss of Jarid2 in Jak2V617F/+ background accelerates the progression of MPN.
To more completely understand the tumor suppressor function of Jarid2 in MPN, we aimed to define its role in normal hematopoiesis. Jarid2 is highly expressed in myeloid-biased compared to lymphoid-biased HSCs, suggestive of a role in HSC subtype determination. Moreover, conditional knock-out studies show that each core component of PRC2 (EED, SUZ12 and EZH2) has distinct as well as overlapping functional properties in hematopoiesis. To study the function of Jarid2 in normal hematopoiesis, we crossed Jarid2fl/fl mice to the Vav-CRE strain to facilitate conditional inactivation of Jarid2 in hematopoietic cells. Vav1-CRE is expressed throughout life in definitive HSCs and all hematopoietic lineages starting at E10.5. Analysis of eight-week old Vav1-CRE:Jarid2fl/fl mice showed that complete loss of Jarid2 induced a significant compromise in hematopoiesis with a robust reduction in phenotypically-defined bone marrow HSCs, a defective B-cell generation in the bone marrow (BM), a differentiation block in T-cell development in thymus, and a significant reduction in peripheral blood counts.
A competitive transplantation strategy was also employed to assess the stem cell potential of Jarid2-KO HSCs. 2.5 x 105 whole bone marrow cells isolated from adult mice were transplanted into lethally irradiated recipient mice along with 2.5x105 whole bone marrow cells from congenic wild-type mice. Peripheral blood analysis of these mice over the period of 16-weeks post-transplant has shown that the loss of Jarid2 disrupts HSC function, leading to enhanced myeloid and reduced lymphoid output.
Collectively, these data illustrate that Jarid2 is required for hematopoietic hemostasis including appropriate lineage fate determination of HSCs. The loss of Jarid2 in a Jak2V617F background promotes acceleration of MPN and implicates Jarid2 as a hematopoietic tumor suppressor.
Kaplan-Meier analysis of a cohort of Jarid2KO-Jak2V617F (n = 5) and Jarid2HET -Jak2V617F (n = 6) and littermate controls (n = 4-8 each).
Kaplan-Meier analysis of a cohort of Jarid2KO-Jak2V617F (n = 5) and Jarid2HET -Jak2V617F (n = 6) and littermate controls (n = 4-8 each).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal