Abstract
Background:
It is accepted that the dramatic historical decrease in mortality from ALL and AML in children and more recently AYAs is directly related to improved participation in NCI sponsored COG clinical trials. It is also known that African-American (AA) and Hispanic children, Hispanic females, and particularly AYAs 15 to 39 years are under-represented in COG clinical trials and may benefit from targeted attention. AA and Hispanic children with ALL and AML have worse survival than white and Asian children even with modern therapy where cure rates have improved drastically. Access to standard accepted chemotherapy for leukemia, socio-economic status and insurance status, differences in disease phenotype and pharmacogenetic variations play a role in these racial and ethnic disparities. AYAs with leukemia have experienced variable improvement in survival over the past two decades due partly to insufficient cancer clinical trial enrollment. Uninsured, older patients and those treated by non-pediatric oncologists were less likely to enroll onto clinical trials. Multiple studies of ALL in North America and Europe have shown AYA patients treated with pediatric "inspired" protocols have better outcomes than AYA patients treated with protocols designed for adults. Enhancing access to quality cancer care in a timely manner in these underrepresented populations (AYA, non white, or under-insured) has emerged as a priority area in oncology. In 2008, to improve access to this largely underserved population, two COG institutions (University of Illinois at Chicago (UIC) and Rush University) and a non-member hospital (John H Stroger Hospital of Cook County) created a unified COG program utilizing one lead IRB and one research team. This study assesses the impact that the collaborative UIC/Rush/Stroger COG program had on clinical trial enrollment for minority underserved and AYA patients with leukemia (ALL and AML).
Methods: A retrospective comparative analyses of COG enrollment data from 2002-2008 and 2008-2014 (pre vs. post-merger) for all patients with ALL and AML by race/ethnicity, age at diagnosis, gender, insurance status, clinical trial type (biology, registry, therapeutic) , and leukemia type was completed. Information regarding the number of COG clinical trials available to enrolment and primary oncologists of enrollees' pre and post merger was collected.
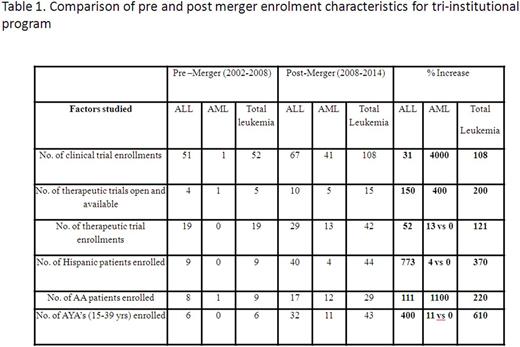
Results: The comparison of the number of patients enrolled pre-merger and post-merger by various variables is shown in table 1.
A total of 40 enrolments with 9 being for therapeutic trials occurred at Stroger Hospital, a site with no access to COG trials prior to the merger. A total of 13 ALL patients and 5 AML patients were enrolled at Stroger Hospital, 7 of whom were uninsured (39%). Nine Pediatric Oncologists, 6 Medical Oncologists and 3 Pediatric nurse practitioners (18 total providers) were engaged in post-merger COG enrollments compared to 6 Pediatric and only 1 Medical Oncologist (7 total providers) engaged pre-merger across the three institutions.
Conclusions: Significant increase in COG leukemia trial availability and enrollment especially for under-represented (non-white, underinsured) minorities and AYAs was a direct result of the creation of the novel UIC/Rush/Stroger COG Clinical Trials program. Cancer clinical trial participation has directly led to improved disease free survival and lower cancer death rates. Collaboration between institutions and Medical and Pediatric Oncologists is critical to participation of AYA's with leukemia in NCI sponsored clinical trials. Improving access to these clinical trials is essential to addressing current disparities in leukemia survival. The UIC/Rush/Stroger COG Program serves as a model for improved collaboration between competing institutions and specialists within institutions to increase access to current clinical trials for minority and AYA patients with leukemia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal